- en Change Region
- Global Site
- Home
- Imaging Applications
- Life Sciences
- Organoid and Organ-on-a-chip Imaging
Organoid and Organ-on-a-chip Imaging
For many years cells cultured in vitro have served as model systems for countless research applications. Cultured cells are kept in suspension or adhering to a vessel surface. However, the physiology of isolated cells is very different from that of complete tissues or organisms. For this reason, various 3D cell culture systems are rapidly gaining in popularity, including organoids, organs-on-a-chip, spheroids, and more. Organs-on-a-chip are particularly promising drug screening models as they can recapitulate key functions of the organ/tissue of interest in a more standardized system. Organs-on-a-chip, and organoids more generally, often allow for the use of multiple cell types in their organization.
Products for Organoid and Organ-on-a-chip Imaging
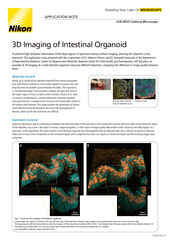
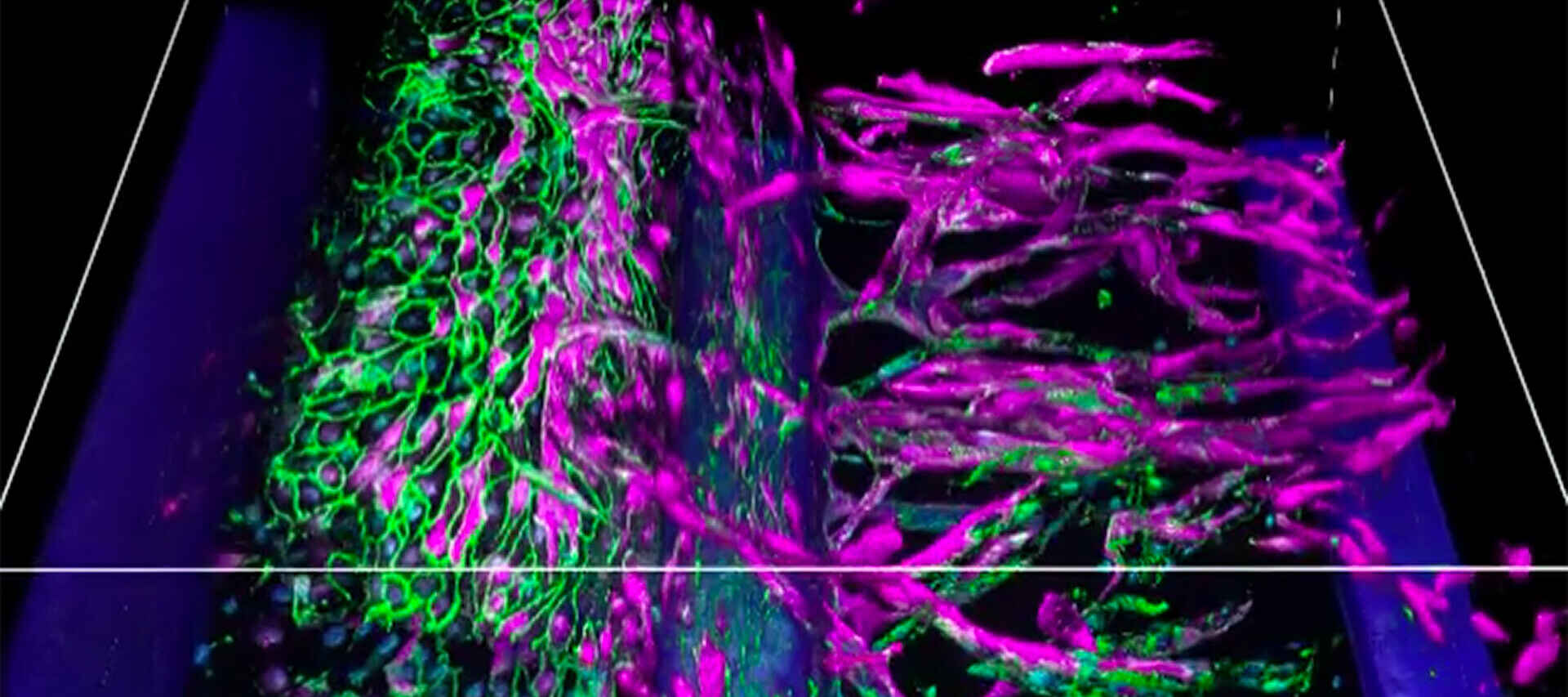
3D image stack of an angiogenesis model cultured using the OrganoPlate® system (MIMETAS).
Nikon’s recently introduced AX / AX R series of confocal microscopes are well-suited for 3D cell culture imaging. The usability of the AX / AX R is improved thanks to the new artificial intelligence (AI)-based Autosignal.ai software module, which identifies optimal confocal settings (e.g., laser power, gain) within seconds. These confocal systems uniquely provide a large 25 mm field of view (FOV) that can be sampled with 8192 x 8192-pixel resolution. The large FOV and high sampling resolution is a powerful combination for imaging large specimens such as organoids at full optical resolution. Additionally, live-sample imaging benefits from the high-speed resonant scanner featured on the AX R model, which allows for fast imaging with up to 2048 x 2048-pixel resolution across the entire 25 mm FOV.
The Yokogawa CSU-W1 spinning disk confocal system is also suited to imaging various types of 3D cell cultures. While spinning disk systems such as the CSU-W1 are more limited in imaging depth than point-scanning systems, the CSU-W1 incorporates an extra-wide pinhole spacing to increase imaging depth compared to other spinning disk confocal models by reducing pinhole crosstalk. The CSU-W1 also features a large ~22.5 mm field of view and an option for a spinning disk with 25 μm diameter pinholes, which allows for imaging with lower magnification objectives and larger FOV while better maintaining confocality.
●: included, ⚬: option
| AX / AX R Confocal Microscopes |
CSU-W1 Spinning Disk Confocal Scanner |
|
|---|---|---|
| Relative Imaging Depth Limit | ~100 – 500 μm | ~50 – 100 μm |
| Detector Type | Multi-alkali and GaAsP-based single element photodetectors | sCMOS and EM-CCD cameras |
| Field of View | 25 mm | ~22.5 mm |
| Available Sample Adapters | AX / AX R | CSU-W1 |
| Emulate Chips | yes | yes |
| Emulate PODs | yes | yes |
| Mimetas Organoplates | yes | yes |
| AIM Biotech Chips | yes | yes |
| Nortis Chips | yes | yes |
| Tissuse Chips | yes | yes |
Related Literature
Discussion of Organoid and Organ-on-a-chip Imaging
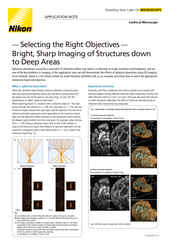
Selecting an Objective Lens for Organoid & Organ-Chip Imaging
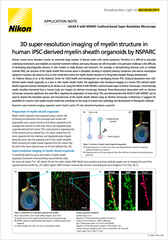
This image is of the functional layer in an Emulate kidney chip (proximal tubule epithelial cells). It was taken with the Nikon CFI S Plan Fluor LWD 20XC objective. The advantages of the objective for this specific system include the long working distance, correction collar, large field of view, and the high NA of 0.70. Without these features it’s difficult to image all the way into the chip at high resolution. This dataset covers the entire bottom and top channels of the chip to provide a complete view of the whole microphysiological system.
As discussed, the primary imaging challenge presented by organoids and other 3D cell culture models is their thickness. A typical adherent cell is about 5 μm thick and can adhere directly to an optical quality glass surface for observation with high numerical aperture (NA) oil immersion objectives. 3D culture models are significantly more difficult to image as they can be tens to hundreds of μm thick, and not always directly attached to a high-quality optical surface.
One of the most important hardware selections is the objective lens. High NA oil immersion objectives may lack the necessary working distance and/or poorly match the refractive index of 3D biological samples (resulting in optical aberrations). To address this concern, Nikon has introduced its Silicone Immersion Series objective lenses, which provide high NA, longer working distances, and improved refractive index matching via silicone immersion. Nikon also manufactures water immersion objectives, which provide similar benefits as silicone immersion objectives. For more detail, see our CFI Apochromat Lambda S objective series.
Many organ-chip and other culture systems necessitate the use of a thick-walled plastic between the objective and the sample. In this case we’re proud to provide the S Plan Fluor LWD objective series. This series features the CFI S Plan Fluor 20XC, which can be corrected for a vessel thickness of up to 1.8 mm while still providing a long working distance of 1.3 – 2.3 mm and a very high NA for this class of 0.70.
Nikon Contract Imaging Services for Organoids and Organs-on-a-chip
The Nikon BioImaging Lab space
The Nikon BioImaging Labs (NBILs) provide contract research services to the biotech and research communities, including remote services* for clients located outside of their local area. Currently, there are NBIL locations in Boston, Massachusetts, USA, Leiden, Netherlands and Shonan, Japan. These Labs have significant experience performing confocal microscopy of organoids and other 3D cell culture systems, including various commercially produced organs-on-a-chip models.
The NBILs are not only capable of data acquisition, but its full-service capabilities also extend to assay design, assay development, assay validation, cell culture, sample preparation, and data analysis. All work is charged on a simple per hour basis to ensure accessibility for all types of customers. If you would like to learn more and see if services at an NBIL are right for you, please don’t hesitate to contact us to set up a free consultation.

* The service may vary depending on the facility. Please contact the Nikon BioImaging Lab near your location for details.
Related Solutions
Glossary
- Available Sample Adapters
- This is a list of manufacturers for whom Nikon manufactures compatible stage adapters to accept their product(s) on our ECLIPSE Ti2 inverted microscope motorized stage.
- Detector Type
- Point-scanning confocal systems use single element photodetectors as signal is detected in a point-wise manner. Spinning disk confocal systems collect data from multiple points in parallel using an array-based detector (camera).
- Field of View
- The field of view of the system, also referred to as the field number, is the diameter of the imaging area at a nominal 1X magnification.
- Relative Imaging Depth Limit
- This indicates the approximate Z (axial) depth range within which the indicated system is able to deliver images with sufficient optical sectioning quality and signal-to-noise. This value can be quite variable and depends heavily on the optical properties of the specimen and vessel, as well as the labeling.
- Home
- Imaging Applications
- Life Sciences
- Organoid and Organ-on-a-chip Imaging