- en Change Region
- Global Site
Application Notes
3D super-resolution imaging of myelin structure in human iPSC-derived myelin sheath organoids by NSPARC
December 2023
Human cranial nerve disorders involve an extremely large number of disease states with varied symptoms. Therefore, it is difficult to elucidate underlying mechanisms and establish an essential treatment method, and many diseases are still intractable. One particular challenge is the difficulty of developing physiologically-relevant, in vitro models to study disease and treatment. For example, in demyelinating diseases such as multiple sclerosis (MS), the structure of the myelin sheath that encases axons is disrupted, resulting in impaired saltatory conduction and various neurological symptoms. However, the absence of an in vitro model that mimics the myelin sheath structure of a living body impedes therapy development.
Dr. Hidenori Akutsu et al. at the National Center for Child Health and Development are developing human iPSC (induced pluripotent stem cell)-derived myelin sheath organoids as a new in vitro myelin sheath model. This application note introduces imaging of a human iPSC-derived myelin sheath organoid structure developed by Dr. Akutsu et al. using the AX/AX R with NSPARC confocal-based super resolution microscope. Conventionally, myelin sheathes harvested from a human body are imaged via electron microscopy. However, three-dimensional observation with an electron microscope consumes significant time and effort, requiring the preparation of many slices. This case demonstrates that AX/AX R with NSPARC can be used to observe the formation process and microstructure of the myelin sheath without using an electron microscope. Furthermore, it suggests the possibility of a novel in vitro myelin sheath model that contributes to the study of cranial nerve pathology and development of therapeutic methods.
Keywords: super-resolution imaging, organoids, myelin sheath, myelin, iPS cells, demyelinating disease, organoids
Preparation of myelin sheath organoids
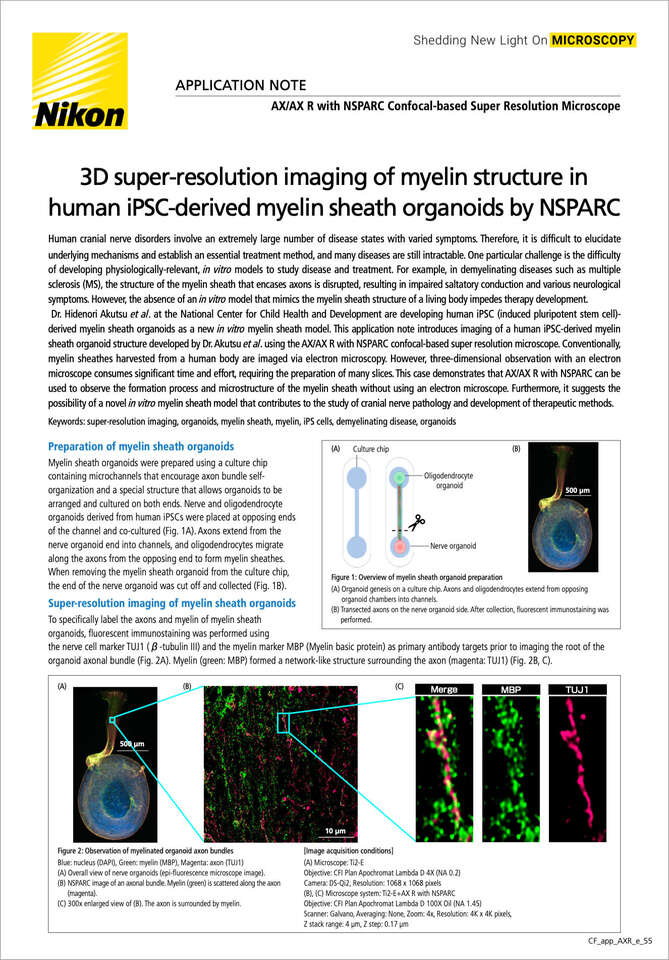
Figure 1: Overview of myelin sheath organoid preparation
(A) Organoid genesis on a culture chip. Axons and oligodendrocytes extend from opposing organoid chambers into channels.
(B) Transected axons on the nerve organoid side. After collection, fluorescent immunostaining was performed.
Myelin sheath organoids were prepared using a culture chip containing microchannels that encourage axon bundle self-organization and a special structure that allows organoids to be arranged and cultured on both ends. Nerve and oligodendrocyte organoids derived from human iPSCs were placed at opposing ends of the channel and co-cultured (Fig. 1A). Axons extend from the nerve organoid end into channels, and oligodendrocytes migrate along the axons from the opposing end to form myelin sheathes. When removing the myelin sheath organoid from the culture chip, the end of the nerve organoid was cut off and collected (Fig. 1B).
Super-resolution imaging of myelin sheath organoids
To specifically label the axons and myelin of myelin sheath organoids, fluorescent immunostaining was performed using the nerve cell marker TUJ1 (β-tubulin III) and the myelin marker MBP (Myelin basic protein) as primary antibody targets prior to imaging the root of the organoid axonal bundle (Fig. 2A). Myelin (green: MBP) formed a network-like structure surrounding the axon (magenta: TUJ1) (Fig. 2B, C).
Figure 2: Observation of myelinated organoid axon bundles
Blue: nucleus (DAPI), Green: myelin (MBP), Magenta: axon (TUJ1)
(A) Overall view of nerve organoids (epi-fluorescence microscope image).
(B) NSPARC image of an axonal bundle. Myelin (green) is scattered along the axon (magenta).
(C) 300x enlarged view of (B). The axon is surrounded by myelin.
[Image acquisition conditions]
(A) Microscope: Ti2-E
Objective: CFI Plan Apochromat Lambda D 4X (NA 0.2)
Camera: DS-Qi2, Resolution: 1068 x 1068 pixels
(B), (C) Microscope system: Ti2-E+AX R with NSPARC
Objective: CFI Plan Apochromat Lambda D 100X Oil (NA 1.45)
Scanner: Galvano, Averaging: None, Zoom: 4x, Resolution: 4K x 4K pixels,
Z stack range: 4 µm, Z step: 0.17 µm
3D observation of myelinated organoid axons
Furthermore, to observe the state of myelin localized near axons in detail, AX/AX R with NSPARC was used for z-stack imaging, and 3D reconstruction was performed using the NIS-Elements imaging software (Fig. 3, Fig. 4). 3D images suggested that myelin (green; MBP) was localized such that it wrapped around the axon (magenta : TUJ1) at regular intervals. It is rare to see detailed 3D observations of the myelin surface because conventional electron microscope images are often used to observe the cross section of the myelin sheath.
Figure 3: 3D images of myelinated organoid axons
(A) Left 45°, (B) Front, (C) Right 45°
Green: myelin (MBP), Magenta: axon (TUJ1)
[Image acquisition conditions]
Microscope system: Ti2-E+AX R with NSPARC
Objective: CFI Plan Apochromat Lambda D 100X Oil (NA 1.45)
Scanner: Galvano, Zoom: 4x, Resolution: 4K x 4K pixels, Averaging: None, Z range: 4 µm, Z step: 0.17 µm
Figure 4: Cropped images of Figure 3 (framed in Figure 3)
(A) Left 45°, (B) Front, (C) Right 45°
Green: myelin (MBP)
Magenta: axon (TUJ1)
Summary
Images of axon bundles of myelin sheath organoids prepared from human iPSCs were acquired using the AX/AX R with NSPARC confocal-based super resolution microscope, and 3D images were constructed by NIS-Elements imaging software. By observing the 3D structure, we were able to clearly capture the presence of myelin in close proximity to the axons.
AX/AX R with NSPARC imaging of myelin sheath organoids by co-culture of nerves and oligodendrocytes introduced in this application note does not require complicated pretreatment as with electron microscopy, and can directly capture wrapping of the myelin sheath around axons. Furthermore, AX/AX R with NSPARC achieves a wide field of view and 3D structures can be easily observed simply by constructing 3D z-stack images using NIS-Elements, without the need for creating slice specimens as in electron microscopy.
The prevalence of neurological diseases is increasing due to increasing longevity and complex environmental factors. Neurological diseases exhibit a wide variety of symptoms because they impart functional abnormalities in all organs and tissues controlled by nerves. Many neurological diseases significantly impair human QOL (Quality of Life), and it is an urgent task to overcome them. Demyelinating diseases caused by damage to the myelin sheath also present various symptoms such as sensory, motor function, and mental disorders, and inevitably lower QOL. There is evidence that an autoimmune reaction is involved in the onset of demyelination, but symptomatic therapy is the only treatment option until the underlying mechanism is identified and causal therapy is developed. A system that can directly evaluate the winding and shape of myelin sheaths on axons is effective for searching for the cause of such demyelinating diseases and evaluating drug candidates. However, an in vitro myelin sheath model suitable for drug evaluation has not yet been established.
Imaging of myelinated organoids by AX/AX R with NSPARC can quantify phenotypic differences between normal and diseased myelinated organoids, activation of myelination before and after drug addition to disease-derived pathological models, and changes in the regularity of myelin sheath arrangement. Therefore, this imaging workflow may contribute to the evaluation of drug efficacy. Furthermore, co-culture with immune cells or direct observation and evaluation of the effects of inflammatory substances is expected to contribute to the elucidation of disease causes and the development of causal therapies.
Acknowledgments
We would like to express our sincere gratitude to Director Dr. Hidenori Akutsu and Dr. Shoko Yoshii of the Department of Reproductive Medicine, Center for Regenerative Medicine, National Center for Child Health and Development, for their great cooperation in preparing and providing samples, as well as numerous advices.
References
Alexander Kister and Ilya Kister, Overview of myelin, major myelin lipids, and myelin-associated proteins. Front Chem., Volume 10 (2022)
Footnote
Myelin sheath
A lipid-rich cell membrane that wraps multiple nerve axons. Up to 100 layers of lipid bilayers overlap, and MBP (myelin basic protein) exists between the layers. It electrically insulates axons, allowing nerve impulses (action potentials) to propagate rapidly along the axons. Damage to the myelin sheath disrupts its insulating function, slowing or impairing the propagation of nerve impulses along the axons, resulting in nerve dysfunction. In addition to multiple sclerosis, there are multiple diseases related to myelin damage and degradation, such as neuromyelitis optical and cognitive decline.
Product information
AX/AX R with NSPARC Confocal-based Super Resolution Microscope
The super-resolution detector NSPARC, which has 25 array detectors, achieves even higher resolution with a high S/N ratio, without impairing the functions of the conventional AX/AX R confocal microscope.
