- en Change Region
- Global Site
- Home
- Imaging Applications
- Clinical Research
- Imaging Fixed Slides
Imaging Fixed Slides
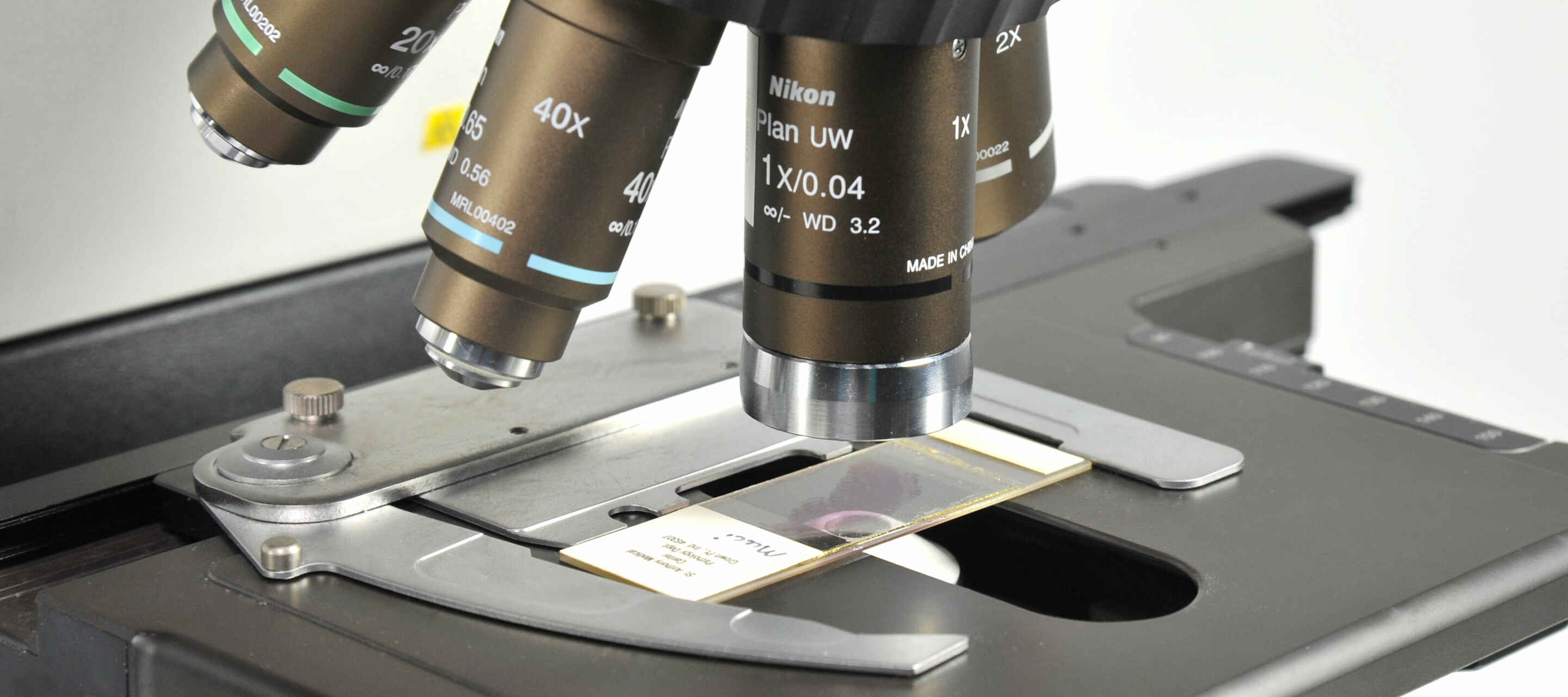
One of the most common applications of the light microscope is imaging fixed specimens mounted on standard size glass microscope slides, including the glass coverslip. While chambered vessels such as multi-well plates have become popular for imaging live samples, glass slides remain the most popular choice for imaging biological samples that have been chemically fixed, and allows for simple long-term storage. Refractive index-tuned mounting media make it straightforward to image at the highest resolution possible with minimal aberration.
Products for Imaging Fixed Slides
The ECLIPSE Ni-E upright motorized microscope is Nikon’s upright motorized microscope system for automated imaging of microscope slides in a research setting. This microscope features Nikon’s proprietary stratum structure, allowing for double-layer mounting of a fluorescence filter cube turret and high-speed emission filter wheel, or other device. Furthermore, it is possible to assign condenser, field stop, aperture stop, and ND filter settings to each objective lens position to help streamline operation.
The Nikon BioPipeline SLIDE high content imaging system is based on the Ni-E microscope and intended for applications such as slide scanning/whole slide imaging of fixed samples. This system includes a Marzhauser Slide Express 2 robotic slide loader, which is able to hold and automatically exchange up to 120 slides. This system is intended for high throughput and high content imaging of fixed samples prepared on standard glass microscope slides.
The ECLIPSE Ci Series of upright microscopes are intended for fixed slide imaging applications in pathology* – designed for repeated use in the clinical laboratory. As with our other upright microscopes, even illumination across the field of view is provided using a fly-eye lens system. The ECLIPSE Si upright microscope provides both imaging and ergonomic features necessary for increasing operational efficiency in both the clinical laboratory and classroom.
Widefield epi-fluorescence imaging can be performed in combination with our D-LEDI Fluorescence LED Illumination System, which provides full visible spectrum coverage using four LED modules (peak wavelengths are 385 nm, 475 nm, 550 nm, and 621 nm). Each of the four LEDs can be individually addressed both in software and hardware-triggered configurations. The D-LEDI is bright, vibration-free, alignment-free, and maintenance-free. Third-party light sources can also be configured on the microscopes listed here (with the exception of the Si).
*The Ci-L plus model upright microscope is not certified for use as a clinical device in all world areas. Please contact your local sales representative for more information.
●: included, ⚬: option
| ECLIPSE Ni-E Upright Motorized Microscope |
ECLIPSE Ni-U Upright Microscope |
ECLIPSE Ci Series Upright Microscopes |
ECLIPSE Si Upright Microscope |
BioPipeline SLIDE High Content Imaging System |
|
|---|---|---|---|---|---|
| Transmitted Light Source | LED or Halogen | LED or Halogen | LED (Ci-E, Ci-L models) Halogen (Ci-S model) | LED | LED or Halogen |
| Motorization | yes | Partial Motorization Available | Partial Motorization Available (Ci-E model only) | no | yes |
| Field of View | 25 mm*1*3 22 mm*2 |
25 mm*4 22 mm*2 |
25 mm*5 22 mm*2 |
22 mm | 25 mm |
| Widefield Fluorescence Light Source | Nikon D-LEDI Illuminator, 3rd party light sources available |
Nikon D-LEDI Illuminator, 3rd party light sources available |
Nikon D-LEDI Illuminator, 3rd party light sources available |
Built-in white light LED (same as used for brightfield observation) | Nikon D-LEDI Illuminator, 3rd party light sources available |
| Compatible Confocal & Multiphoton Systems | ECLIPSE Ni-E | ECLIPSE Ni-U | ECLIPSE Ci Series | ECLIPSE Si | BioPipeline SLIDE |
| AX / AX R Point-Scanning Confocal Systems | yes | no | no | no | no |
| A1 MP+ / A1R HD MP+ Point-Scanning Multiphoton Systems | yes | no | no | no | no |
| Yokogawa CSU-X1 Spinning Disk Confocal | yes | yes | no | no | no |
| Yokogawa CSU-W1 Spinning Disk Confocal | yes | yes | no | no | no |
| Compatible Contrasting Techniques | ECLIPSE Ni-E | ECLIPSE Ni-U | ECLIPSE Ci Series | ECLIPSE Si | BioPipeline SLIDE |
| Brightfield | yes | yes | yes | yes | yes |
| Confocal - Point-Scanning | yes | no | no | no | no |
| Confocal - Spinning Disk | yes | yes | no | no | no |
| Darkfield | yes | yes | yes | yes | yes |
| Differential Interference Contrast (DIC) | yes | yes | no | no | yes |
| Multiphoton | yes | yes | no | no | no |
| Phase Contrast | yes | yes | yes | yes | yes |
| Simple Polarized Light | yes | yes | yes | yes | yes |
| Widefield Dia-Fluorescence | no | no | no | yes | no |
| Widefield Epi-Fluorescence | yes | yes | yes | no | yes |
*1 with NI-TT-E motorized quadrocular tilting tube, NI-TT quadrocular tilting tube, C-TT trinocular tube, C-TF trinocular tube
*2 with C-TE2 ergonomic binocular tube, C-TB binocular tube
*3 AX / AX R Confocal
*4 with NI-TT quadrocular tilting tube, C-TT trinocular tube, C-TF trinocular tube
*5 with C-TT trinocular tube, C-TF trinocular tube
Discussion of Imaging Fixed Slides
Choosing the Right Objective Lens for Imaging Fixed Slides
Proper choice of objective lens is just as important for imaging of fixed microscope slides as for other imaging applications. One of the greatest advantages of imaging fixed slides is the diversity of mounting media options, which vary widely in formulation and refractive index (RI), allowing for precise tuning of imaging conditions. This minimizes spherical aberration and allows for deeper imaging while maintaining high resolution performance. Modern mounting media generally include anti-fade reagents to support extended imaging of fluorescently labeled specimens.
Nikon manufactures a variety of oil immersion lenses that are RI-matched to several common mounting media. The CFI Plan Apochromat Lambda D Series objectives includes a range of dry and oil immersion objectives suited towards imaging fixed slides, including the CFI Plan Apochromat Lambda D 100X Oil lens (NA = 1.45). For users requiring the highest possible numerical aperture (NA) and are imaging very thin samples, the CFI Apochromat TIRF Series objectives are here to help. Both the 60X and 100X oil immersion TIRF objectives feature an NA of 1.49.
Choosing the Right Contrasting Technique(s) for Imaging your Slides
Different contrasting techniques predominate depending on the application. Imaging fixed hematoxylin and eosin (H&E) stained pathology samples is performed almost exclusively using brightfield microscopy. In clinical research, fluorescence imaging is more common. Widefield fluorescence imaging combined with deconvolution or deblurring is often sufficient for characterization of samples up to ~20 micrometers (μm) thick. However, thicker samples may require a more intensive optical sectioning technique, such as confocal or multiphoton.
Phase-based transmitted light techniques such as phase contrast and differential interference contrast (DIC) are popular choices for imaging otherwise-transparent samples (e.g. fixed cells) with improved contrast compared to brightfield and without using specific labels. DIC provides optical sectioning and the highest possible resolution of such techniques. Phase contrast has the benefit of lower cost and is easier to implement.
Brightfield image of an H&E-stained mouse embryo gut tissue section acquired using a CFI Plan Apochromat Lambda D 20X objective lens.
Breast cancer, FISH method (Objective: CFI Plan Apochromat Lambda D 100X Oil)
Photo courtesy of: St. Marianna University Hospital
Diphyllobothrium latum Egg - Unstained (Objective: CFI Achromat DL 10X, Camera: Digital Sight 1000)
Different Cameras for Different Applications
Proper selection of camera system (or other detector) goes hand-in-hand with selection of appropriate contrast technique(s). Brightfield imaging of samples labeled with absorption-based stains (such as H&E slides) are typically imaged using a color camera. Conversely, detection of (often weak) fluorescence signals is generally performed using a monochrome camera, which provides higher sensitivity. Point-scanning confocal and multiphoton techniques use dedicated single-element detectors, such as photomultiplier tubes (PMTs), instead of cameras, which are composed of large multi-pixel arrays.
There are also different camera types to choose from beyond color or monochrome. Charge-coupled device (CCD) cameras once dominated the market, and are still suited to various applications, but have largely been overtaken in the market by complementary metal oxide semiconductor (CMOS)-based cameras, which provide greater pixel number and faster acquisition rates (supporting the imaging of larger fields of view).
Nikon’s current Digital Sight (DS) series cameras utilize CMOS technology. The Digital Sight 100 color CMOS camera is suitable for applications that require high color reproduction for color image acquisition. The Digital Sight 1000 color CMOS camera is the most cost-effective microscope camera offered by Nikon, but still supports video rate (30 frames per second) full HD (1920 x 1080 pixels) recording, and using the same proprietary color reproduction algorithms as other Nikon color cameras. The Digital Sight 10 color CMOS camera features a full-frame CMOS image sensor, supporting a large field of view (FOV) of up to 25 mm in combination with various Nikon microscope stands. The DS-Qi2 monochrome CMOS camera is intended for fluorescence imaging applications, and also supporting the large 25 mm FOV.
Fluorescence image of Indian muntjac deer skin fibroblasts labeled with Alexa Fluor™ 488 – Phalloidin and imaged using the Nikon DS-Qi2 monochrome CMOS camera.
Stomach, SMA staining, 17x12 Tiled images (Objective: CFI Plan Apochromat 40XC, Camera: Digital Sight 10)
Photo courtesy of : Nichirei Biosciences Inc.
Photos courtesy of: Kazuhiro Muraoka, Photography Division, Tokyo Women's Medical University
Glossary
- Compatible Confocal & Multiphoton Systems
- Advanced optical sectioning techniques based on confocal and multiphoton imaging are useful for imaging discrete 2D sections in relatively thick samples, up to several hundred micrometers for confocal and 1 millimeter or more for multiphoton.
- Compatible Contrasting Techniques
- Light microscopy systems are typically capable of a transmitted light technique, such as phase contrast or DIC, and often a fluorescence imaging modality, such as widefield or confocal.
- Field of View
- The field of view of the system, also referred to as the field number, is the diameter of the imaging area at a nominal 1X magnification.
- Motorization
- This refers to the motorization of standard system components, including the XYZ stage, fluorescence filter cube turret, objective nosepiece, and more.
- Transmitted Light Source
- The most common types of illuminators for transmitted light (diascopic) imaging are light emitting diodes (LEDs) and halogen lamps. LEDs are bright, more energy efficient and last longer than halogen lamps.
- Widefield Fluorescence Light Source
- Widefield fluorescence imaging microscopes most commonly now employ LEDs, which have largely replaced Mercury arc and metal halide lamps, which are energy intensive and require frequent bulb changes.
- Home
- Imaging Applications
- Clinical Research
- Imaging Fixed Slides
