Modular illumination system with ultimate flexibility and expandability.
The Nikon Ti2-LAPP system provides modular illuminators for total internal reflection fluorescence (TIRF), photoactivation/conversion, photobleaching, epi-fluorescence, as well as super-resolution microscopy (N-STORM). Each module can be flexibly combined to build microscope systems that are optimized for individual research needs. For example, multiple TIRF modules can be incorporated into a single microscope for anisotropy experiments and fast, multi-angle TIRF imaging. Combined with the Ti2’s stratum structure, up to five illumination modules can be incorporated into a single microscope (e.g. two TIRFs, a FRAP, a DMD, and an Epi-FL module can all be integrated into one Ti2).

Key Features
Fully automated H-TIRF module
Fully automated TIRF adjustment and observation is now possible
The ideal incident angle and focus of the laser for TIRF observation vary depending on specimen and observation conditions. Adjusting the incident angle and focus for achieving TIRF requires skill and experience. The H-TIRF module automatically adjusts the focus and incident angle of the laser for TIRF observation by monitoring the reflection beam. This automatic laser focus adjustment and incident angle adjustment is carried out by the auto-alignment function in NIS-Elements software. Incident angles and penetration depths of the evanescent fields can be saved and reproduced for subsequent experiments to ensure consistent imaging results.


An in vitro preparation of fluorescently-labeled microtubules (tetramethylrhodamine and Alexa 647) and tubulin binding proteins (Alexa 488) was imaged in three different wavelengths using the H-TIRF illuminator and the gradation ND filter. Incident angles can be automatically adjusted for multiple wavelengths.
Image courtesy of Melissa Hendershott and Dr. Ron Vale, University of California, San Francisco
Without the gradation ND filter, TIRF illumination displays a Gaussian profile in the FOV with the center being brightest.
Using the gradation ND filter, a very even TIRF illumination is achieved.

Gradation ND filter out

Gradation ND filter in
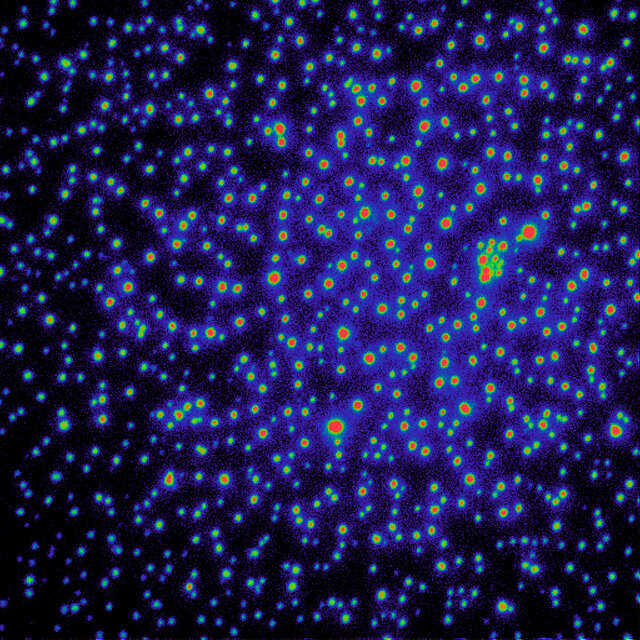
Gradation ND filter out
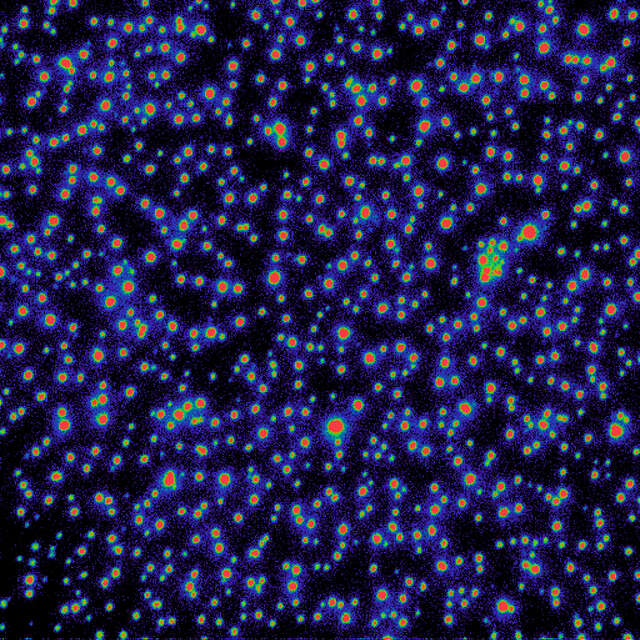
Gradation ND filter in
Motorized TIRF Module
The incident angle of the laser and corresponding penetration depth of the evanescent field can be controlled via NIS-Elements software. When multiple TIRF modules are mounted (see image), the penetration depth can be independently set for each wavelength.


Manual TIRF module
For observation of cell membrane dynamics and single molecules
The manual TIRF module includes a gradation ND filter (similar to the H-TIRF module), enabling even TIRF illumination across the field of view. Using high-sensitivity cameras, one can image single molecules and dynamics of proteins in and near the cell membrane using this TIRF illuminator.

N-STORM module
Achieves a resolution 10 times greater than conventional optical microscopy
Equipped with illumination field (1x, 2x, 4x) motorized switching, as well as an auto-alignment function, this module enables N-STORM super-resolution imaging with the Ti2-LAPP system. This delivers an incredible image resolution of approximately 20 nm, which is 10 times or greater than the limit in conventional optical microscopy. Utilizing STochastic Optical Resolution Microscopy (STORM), it is possible to gain insight into protein-protein interactions at a molecular level.

DMD module
Achieves simultaneous multipoint photoactivation
The DMD module enables photoactivation and photoconversion of user-specified patterns and positions, whereas the conventional FRAP unit only enables photoactivation of a single, manually positioned spot. The DMD illumination shape, size, position, and number can be freely customized using the NIS-Elements software. This capability allows researchers to optically mark a subset of cells or protein populations within a single cell or multiple cells to track their behavior. The DMD module is also optimally suited for optogenetics experiments in which highly customized ROIs can be used to optically induce functional changes in subsets of cells or protein populations. The DMD module can be used with either laser illumination or less phototoxic LED illumination.

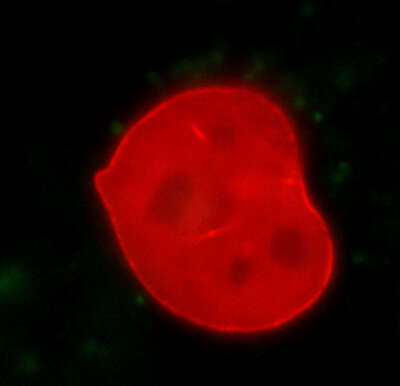
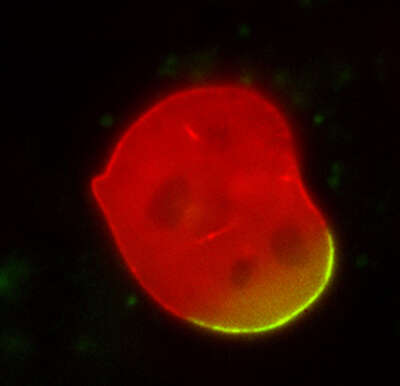
Photoactivation
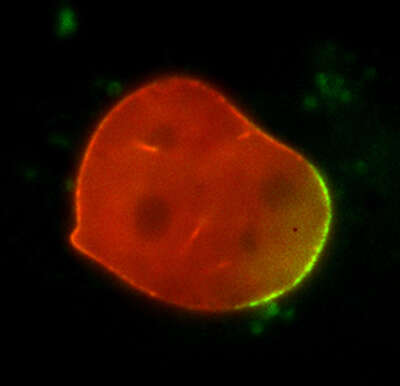
50 mins
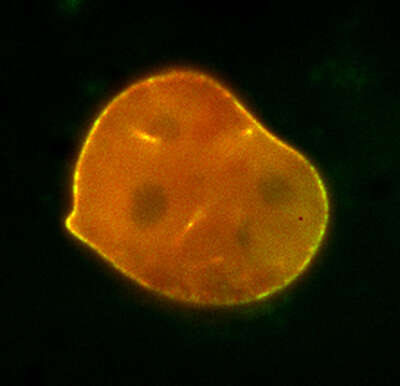
63 mins
A mouse embryonic fibroblast co-expressing mCherry-tagged lamin A (red) and photo-activatable GFP-tagged lamin A was photo-converted (green) in the lower right region using the DMD module and 405 nm LED light. Time-lapse images were captured using the epi-fluorescence illuminator. By photoactivating a sub-population of the lamin proteins, one can observe their dynamics and subunit-exchange behavior.
Image courtesy of Drs. Takeshi Shimi and Bob Goldman, Northwestern University Medical School
FRAP module
For analysis of intracellular-protein dynamics
With this FRAP module, photobleaching and photoactivation/conversion experiments are possible, and with high-frame-rate, high-sensitivity cameras. This module can spot-illuminate a target position in the cell, providing a cost-effective means for the study of intracellular protein dynamics without the use of a point-scanning confocal microscope.


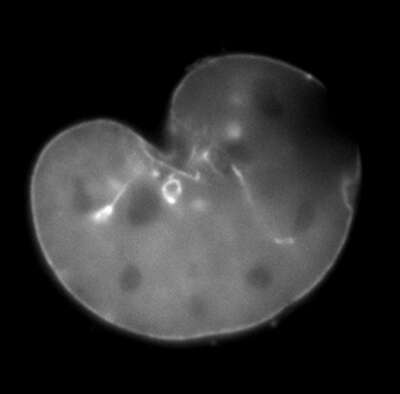
Bleach
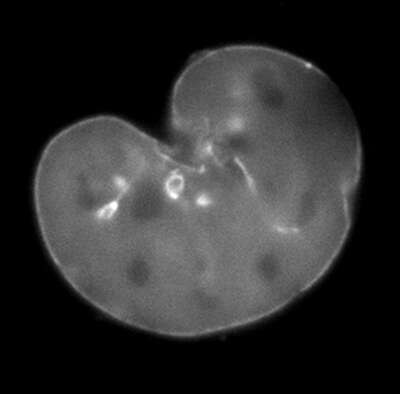
3 mins
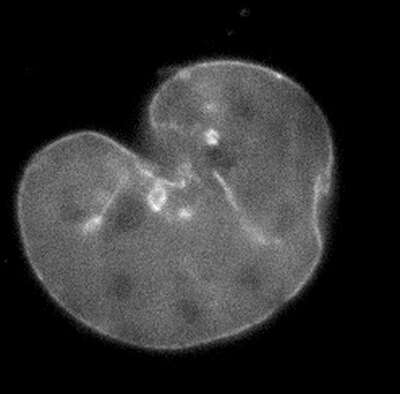
12 mins
A mouse embryonic fibroblast expressing mCherry-lamin A was spot-photobleached in the upper right corner of the nucleus using the FRAP module to study the dynamics of lamin A molecules. Time-lapse images were acquired using the epi-fluorescence illuminator.
Image courtesy of Drs. Takeshi Shimi and Bob Goldman, Northwestern University Medical School
XY galvo scanning module
For simultaneous photostimulation and confocal imaging
The XY galvano scanning unit is a photostimulation module that allows the user to stimulate the desired area of a sample through point scanning by means of laser. When mounted on the Ti2-E inverted microscope together with the AX/AX R confocal system, it allows acquisition of confocal images of live-cell dynamics while simultaneously carrying out photostimulation within a sample. In addition, the unit can be mounted on the AX R MP multiphoton confocal microscope.
*The unit can only be used with the AX/AX R or AX R MP.

Large FOV epi-fluorescence module
Perfect for fluorescence imaging with cameras with large sensors
The EPI FL Module for Large FOV is a basic epi-fluorescence illuminator for the Ti2-LAPP system, and is designed for large FOV imaging applications using Ti2 inverted microscopes. It delivers an unparalleled, large 25mm field of view during epi-fluorescence observation, even though it has a compact design. It is equipped with a quartz fly-eye lens to ensure uniform intensity over the entire field of view and provides high transmittance across a broad spectrum, including UV.

Flexible module combination
The Ti2-LAPP system’s modularity and flexible configuration capability provide custom imaging solutions for individual research needs. Modules can also be easily exchanged or added to adapt to changing experimental needs, an important feature for labs with evolving research directions and multi-user core facilities. For example, by adding a second TIRF module to a single-TIRF configuration, users can easily carry out anisotropy experiments and fast, multi-angle TIRF experiments. Adding a photoactivation/conversion module such as the DMD or FRAP module enables tracking of protein sub-populations, providing insights into protein behaviors that would otherwise be illusive when imaging the entire population.

Two-tiered configuration
Taking advantage of the Nikon Ti2’s stratum structure, modules can be incorporated as two separate layers with multiple modules per layer. Using a dual layer configuration enables optimal filter configuration for each illumination module. For example, by placing the H-TIRF module in the lower layer and a DMD module in the upper layer, separate filter cubes specific for TIRF imaging and photoactivation can be simultaneously used in their respective filter turrets, also residing in the lower and upper layers. This configuration enables optimal filter selection and improves experimental accuracy whilst maintaining the highest acquisition speeds.

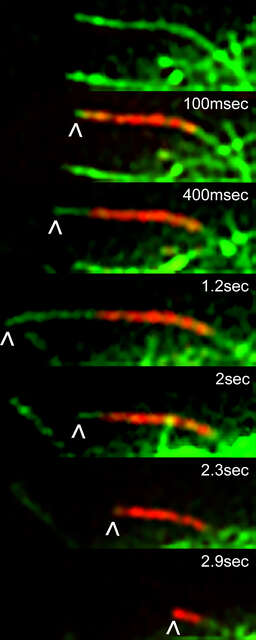
A Drosophila S2 cell expressing EOS-tubulin. The end of a single microtubule was photoconverted using the DMD module and 405 nm LED light. Time-lapse images in dual color TIRF were acquired using the H-TIRF illuminator. The addition of unconverted, green tubulin to the growing end of the photoconverted red microtubule, and shortening (and eventual disappearance) of the photoconverted segment demonstrate the dynamic instability property of microtubules. Arrowheads mark the growing and shrinking end of the photoconverted microtubule.
Image courtesy of Drs. Nico Stuurman and Ron Vale, University of California, San Francisco