- fr Change Region
- Global Site
- Accueil
- Applications
- Sciences de la vie
- Drug Discovery
Drug Discovery
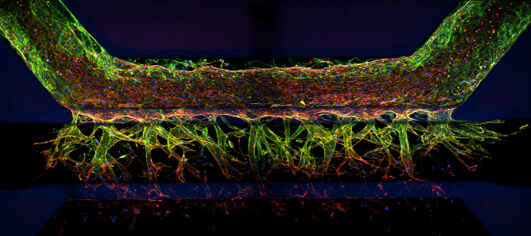
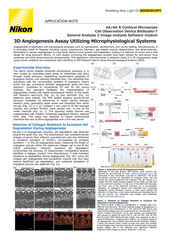
High throughput screening/imaging has occupied a central position in drug discovery testing over the past couple decades – providing automated and highly quantitative options for screening large numbers of candidate compounds for hits. Furthermore, imaging complements genomics, proteomics, and other -omics type methods by providing a spatial context. Combined with advanced techniques such as cell phenotype profiling using artificial intelligence, light microscope-based imaging/screening seems poised to become an even more important (and accessible) component of the drug discovery process. Nikon is committed to providing microscopy-based product solutions to fit these needs.
Produits pour le Drug Discovery
Inverted Microscope Systems
ECLIPSE Ti2-E / Ti2-LAPP: Synchronous operation of the Ti2-E inverted microscope with Ti2-LAPP illuminator modules and other hardware devices allows for complex multidimensional image acquisitions. Additionally, the Ti2-E features the Perfect Focus System 4 (PFS4) focus-locking system, providing the highest level of stability for extended imaging.
Confocal Imaging
AX / AX R: The AX / AX R point-scanning confocal systems are capable of imaging over a wide 25 mm field of view with up to 8192 x 8192-pixel resolution. The AX R is equipped with a resonant scanner capable of very high-speed imaging (2048 x 512 pixels, 1024 x 512 pixels: 30 frames per second), supporting high-throughput screening, live cell imaging, and more.
CSU Series Spinning Disk Confocal Systems: The CSU-W1, and CSU-W1 SoRa spinning disk confocal from Yokogawa can all be configured on the BioPipeline PLATE and LIVE systems, providing flexible, live cell friendly confocal options for high content imaging.
Multiphoton Imaging
AX R MP: The AX R MP is Nikon’s latest multiphoton system, providing deep imaging up to 1.4 mm into the sample using 1300 nm excitation light, making it ideal for late in vivo and intravital imaging. Two dedicated upright microscope stands are available for use with the AX R MP – the single stand for wide samples and the gate stand for deep ones.
Software and AI
NIS-Elements Software: NIS-Elements is Nikon’s flagship, integrated software solution for microscope operation, image analysis, and visualization. NIS-Elements HC is optimized for high-throughput and high-content imaging applications. Heatmaps, sample images, binary masks, assay results, and more can be centrally managed for rapid filtering and analysis. Additionally, the NIS.ai deep learning-based software modules can be incorporated to harness the power of artificial intelligence (AI) for analyses such as image segmentation.
●: Inclus, ⚬: Optionnel
| Base System | Modules | ||||||
|---|---|---|---|---|---|---|---|
| ECLIPSE Ti2-E Inverted Microscope (Widefield Imaging) |
ECLIPSE Ji Digital Inverted |
AX R Resonant Confocal System |
Ti2-LAPP E-TIRF Illuminator |
AX R MP Multiphoton Microscope System |
CSU-W1 Spinning Disk Confocal Scanner |
CSU-W1 SoRa Spinning Disk Super-Resolution System |
|
| Relative Imaging Depth Limit | ~ 5 μm ~ 15 – 25 μm (with deconvolution) |
~ 5 μm ~ 15 – 25 μm (with deconvolution) |
~ 100 – 500 μm | ~ 100 – 300 nm | ~ 500 μm – 1.5 mm | ~ 50 – 100 μm | ~ 50 – 100 μm |
| Supports Video Rate Imaging | ●*1 | ●*1 | ●*2 | ●*1 | ●*2 | ●*3 | ●*3 |
| Field of View | 25 mm diagonal (circular) | 25 mm diagonal (square) | 25 mm diagonal (square) | ~ 10 mm diagonal (circular) | 22 mm diagonal (square) | 17 x 16 mm (rectangular) | 17 x 16 mm (rectangular) |
Supported Imaging Modalities
| ECLIPSE Ti2-E |
ECLIPSE Ji |
AX R | Ti2-LAPP | AX R MP | CSU-W1 | CSU-W1 SoRa |
|
|---|---|---|---|---|---|---|---|
| Brightfield | Yes | Yes | No | No | No | No | No |
| Confocal | No | No | Point-Scanning | No | Point-Scanning | Spinning Disk | Spinning Disk |
| Darkfield | Yes | No | No | No | No | No | No |
| DIC | Yes | No | No | No | No | No | No |
| Nikon Advanced Modulation Contrast (NAMC) | Yes | No | No | No | No | No | No |
| Phase Contrast | Yes | No | No | No | No | No | No |
| Super-Resolution | No | No | ISM with NSPARC detector | No | No | No | Optical Pixel Reassignment |
| TIRF | No | No | No | Yes | No | No | No |
| Volume Contrast | Yes | Yes | No | No | No | No | No |
| Widefield Fluorescence | Yes | Yes | No | No | Yes | No | No |
Compatible Microscope Stands
| ECLIPSE Ti2-E |
ECLIPSE Ji |
AX R | Ti2-LAPP | AX R MP | CSU-W1 | CSU-W1 SoRa |
|
|---|---|---|---|---|---|---|---|
| ECLIPSE Ti2 Series Inverted |
No | No | Ti2-E | Ti2-E | Ti2-E | Yes | Yes |
| ECLIPSE Ji Digital inverted |
No | No | Yes | No | No | No | No |
| ECLIPSE Ni Series Upright | No | No | Ni-E | No | No | Yes | No |
| ECLIPSE FN1 Upright |
No | No | Yes | No | No | Yes | No |
| AX-FNGP Upright | No | No | No | No | Yes | No | No |
| AX-FNSP Upright | No | No | No | No | Yes | No | No |
*1 Limited by camera system
*2 30 FPS with 512 x 512 scan
*3 Limited by camera system and disk rotation speed
Littérature reliée
Discussion sur le Drug Discovery
Selecting a Microscope System for your Drug Discovery Model
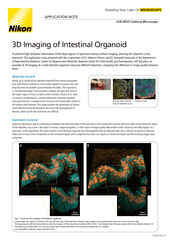
Intestinal organoid stained for DNA (blue; DAPI), mucin (green; produced by goblet cells), and somatostatin (red; produced by enteroendocrine cells), imaged using point-scanning confocal microscopy.
Model systems for drug discovery run just about the entire range of possibility, from in vitro adherent cell cultures to whole model organisms and almost everything in between. Complex 3D cell culture models such as spheroids, organoids, and organs-on-chips can be composed of multiple cell types to better recapitulate various physiological characteristics lost in traditional adherent cell cultures, which are the traditional standard for high throughput screening. Additionally, organoids can even be cultured from autologous (patient-derived) cells for developing precision medicines.
Widefield fluorescence imaging is an appropriate choice for relatively flat samples, such as adherent cells cultured in multi-well plates, the traditional standard for high throughput screening. It is fast, sensitive, and cost effective, but does not provide intrinsic optical sectioning – the ability to effectively image a single plane in a thick 3D specimen.
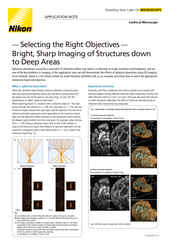
Physically larger 3D models, such as organoids and tissue, may require an imaging technique with optical sectioning capabilities to capture the features of interest without being overly compromised by out-of-focus blur. Confocal microscopy is the standard for such applications, Nikon offers the AX / AX R confocal microscopes, point-scanning systems for imaging discrete sections up to several hundred micrometers deep in various samples.
While confocal imaging is a suitable choice for optical sectioning at depth, sometimes it is insufficient. In vivo imaging in the presence of thick and scattering tissues often necessitates the use of multiphoton imaging, such as the Nikon AX R MP multiphoton microscope system, which uses multiphoton excitation with near-infrared to infrared light to minimize out-of-focus excitation and scattering.
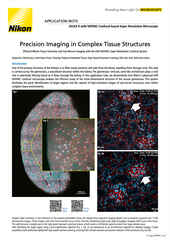
Quantitative Phase Imaging for Cell Analysis

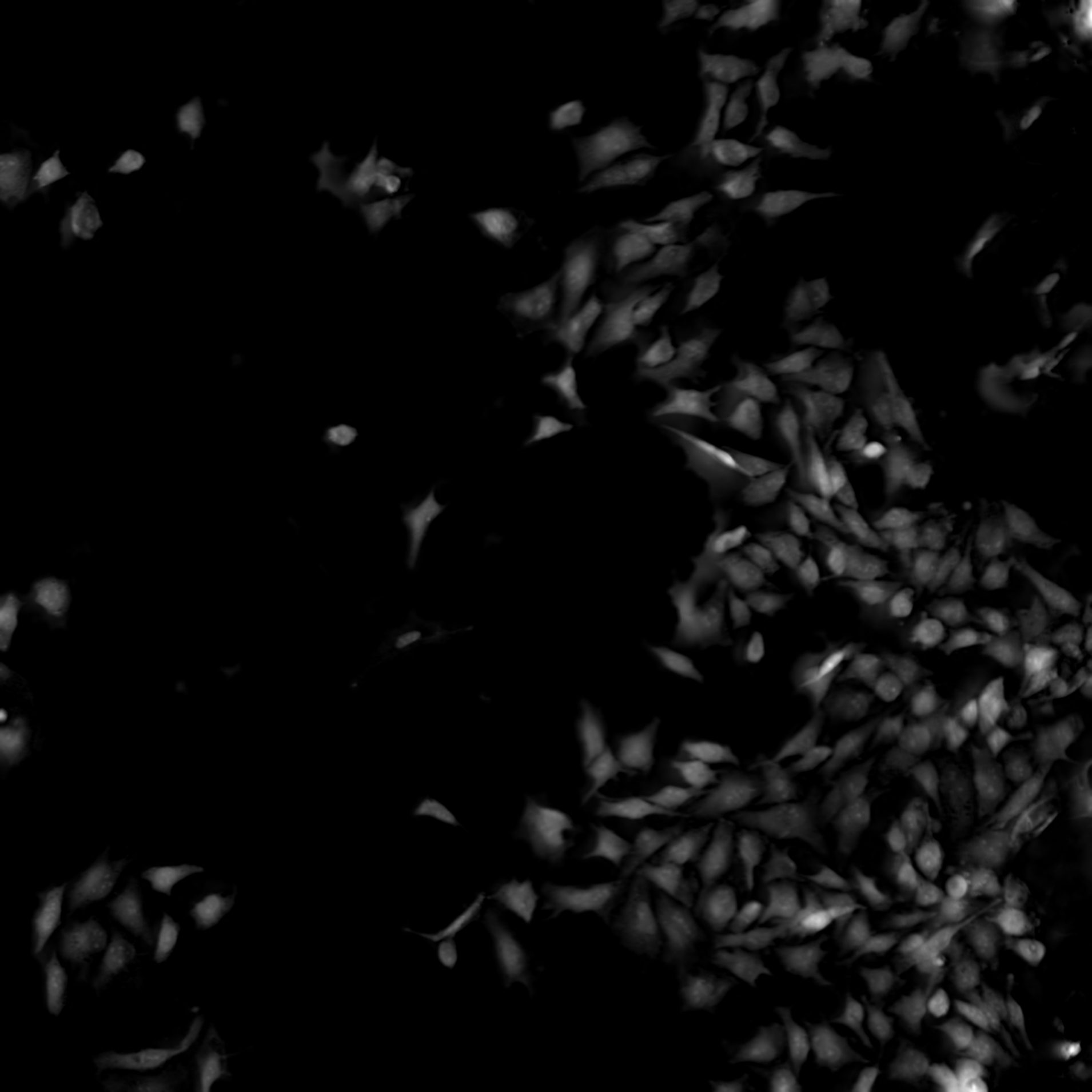
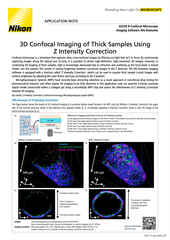
Nikon’s ECLIPSE Ti2-E inverted microscope, which forms the base of the BioPipeline LIVE and BioPipeline PLATE systems, is capable of quantitative phase imaging via the Nikon Volume Contrast technique, which only requires a small Z stack of brightfield images (as few as three images per stack) to create the phase distribution image. The resulting phase distribution image is brightest where the optical path difference is greatest – in the middle of the cell when imaging a culture. This is beneficial for cell segmentation via thresholding and other methods.
Unlike other transmitted light (diascopic) imaging techniques, such as phase contrast, Volume Contrast is unaffected by the meniscus effect, which can be significant in well plates due to the small diameter of individual wells. Performance of phase contrast and Volume Contrast in the presence of a meniscus is compared in the image on the right.
For more information, see our recent application note detailing the use of Volume Contrast as part of a label-free cell proliferation assay, a commonly performed assay in drug discovery research.
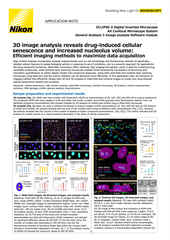
Enhancing Drug Discovery Research using Artificial Intelligence
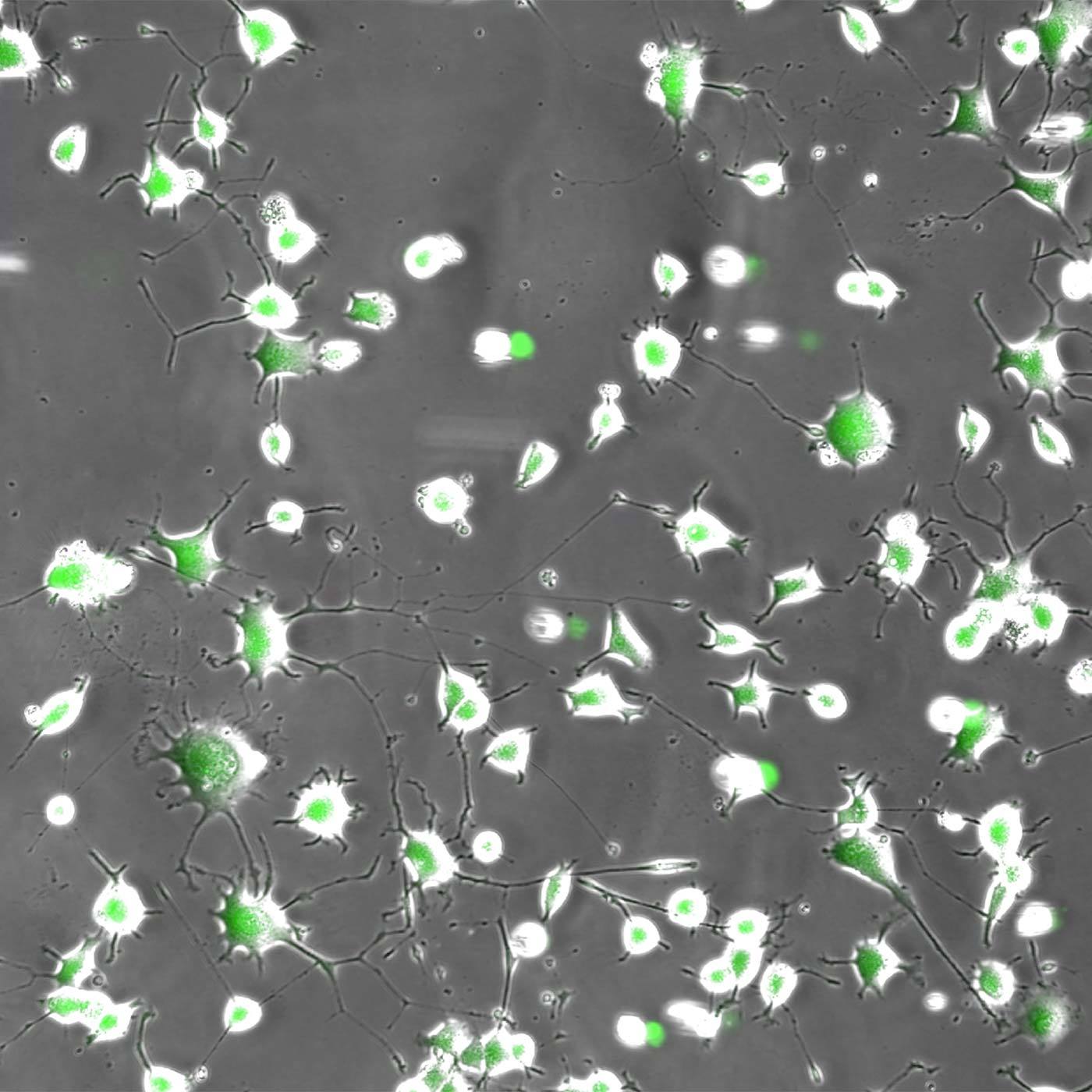
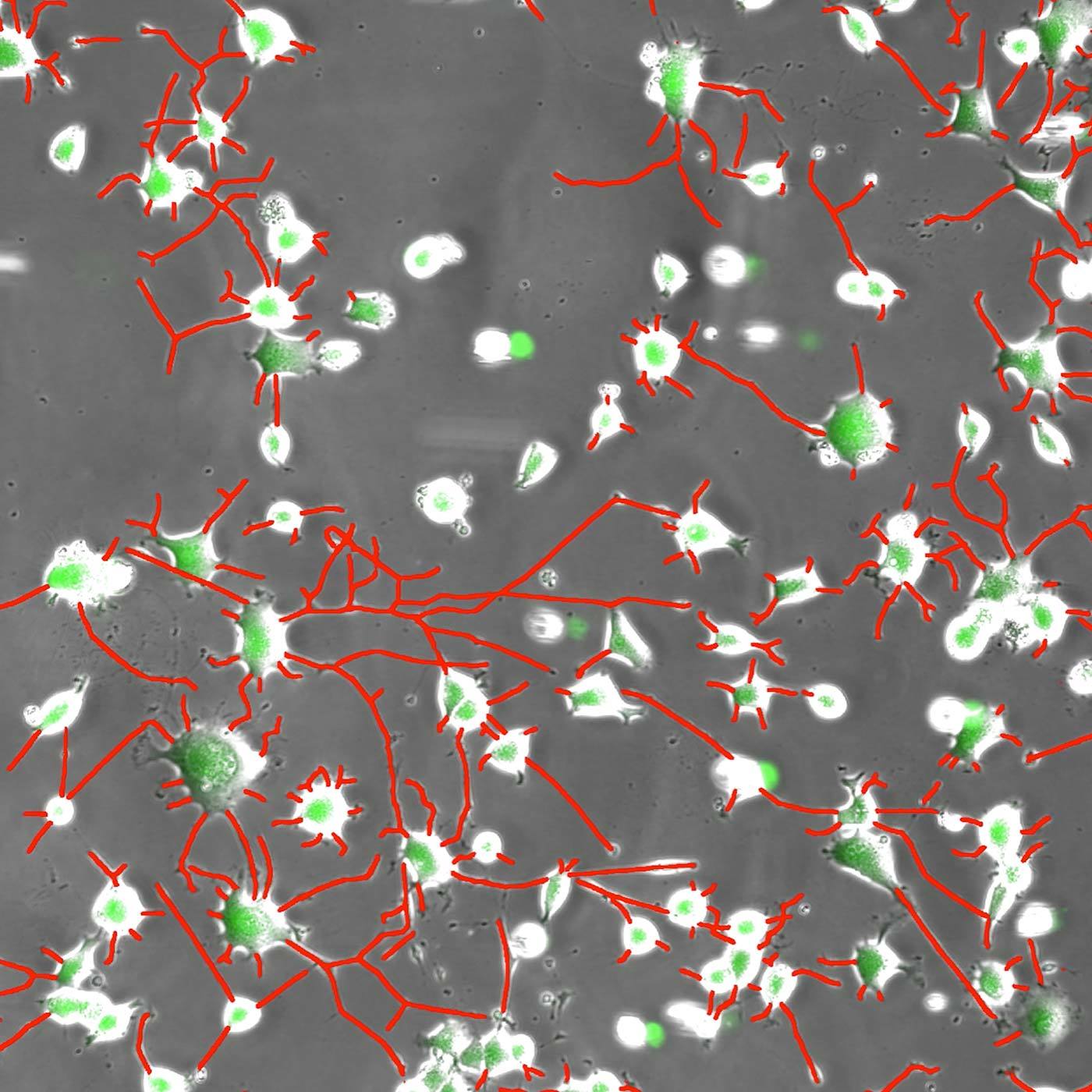
Neurites in phase-contrast were not possible to define accurately by traditional thresholding. Segment.ai was trained on hand-traced neurites (human recognized) and learned how to trace neurites in subsequent images.
One of the great advantages of image-based assays is their rich information content. Until recently, however, relatively little of this information could be leveraged in a practical manner. However, new approaches based on artificial intelligence (AI), and particularly deep learning (DL) methods using artificial neural networks (ANNs), allow for deeper correlations between image features to be inferred and applied towards characterizing morphology and phenotype. Such analytical approaches have been referred to as “cell profiling” or “image-based profiling,” and represent a very active area of development.
In addition to profiling, DL can be used to help accelerate and strengthen image analyses in other ways. Nikon is committed to the design of reliable DL-based image analysis tools under the umbrella of NIS.ai – a series of software modules available for the NIS-Elements software. For example, the Segment.ai module can be trained to automatically segment difficult image features that are difficult to isolate using classical approaches.
Other NIS.ai modules of potential application for drug discovery work includes Convert.ai, which can be trained to predict image features from a fluorescence channel using only a brightfield or another transmitted light channel as a reference, which can help cut down on both cytotoxicity (from the fluorescent label) and phototoxicity (from the high intensity light used for fluorescence imaging). Relatedly, the Enhance.ai module can be trained to predict a higher signal-to-noise version of noisy image data. This allows for a reduction in illumination intensity, and thus phototoxicity.
High throughput widefield fluorescence imaging of larger model systems, such as organoids, can benefit from our Clarify.ai module, which is pre-trained to provide automatic blur removal from widefield fluorescence microscopy images. This allows for users to benefit from the speed of widefield fluorescence imaging, but with enhanced optical sectioning and without the need for a confocal system.
Nikon Contract Imaging Services for Pre-Clinical Drug Discovery Research
Nikon contract research services at a glance.
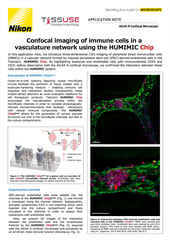
The Nikon BioImaging Labs provide contract research services to their local biotech and research communities, as well as remote services* for clients located elsewhere. With locations in Cambridge, MA, USA, Leiden, The Netherlands, and Shonan, Japan, the Nikon BioImaging Labs have significant experience working with customers in the biopharma research space and performing confocal microscopy of organoids and other 3D cell culture systems now used for drug discovery studies, including various commercially produced organ-on-chip models.

The Nikon BioImaging Labs are not only capable of data acquisition, but their full-service capabilities also extend to assay design, assay development, assay validation, cell culture, sample preparation, and data analysis. If you would like to learn more and see if services at one of the Nikon BioImaging Labs is right for you, please don’t hesitate to contact us to set up a free consultation.
* The service may vary depending on the facility. Please contact the Nikon BioImaging Lab near your location for details.
Related Solutions
Glossaire
- Drug Discovery
- Drug discovery is an interdisciplinary field focused on the identification and pre-clinical testing of potential medications in vitro, ex vivo, and in vivo. This includes primary assays of the drug’s pharmacological properties, as well as secondary assays of absorption, distribution, metabolism, elimination, and toxicology (ADMET) and related safety factors that are evaluated in the discovery process.
- Field of View
- The field of view of the system, also referred to as the field number, is the diameter of the imaging area at a nominal 1X magnification.
- Relative Imaging Depth Limit
- This indicates the approximate Z (axial) depth range within which the indicated system can deliver images with sufficient optical sectioning quality and signal-to-noise. This value can be quite variable and depends heavily on the optical properties of the specimen and vessel, as well as the labeling.
- Supported Imaging Modalities
- This refers to the various microscopy imaging techniques provided by each system. Note that almost all the imaging modalities supported by the ECLIPSE Ti2-E inverted microscope can still be accessed when it is used as a base for any of the other systems listed in this table.
- Supports Video Rate Imaging
- “Video rate” is traditionally defined as about 30 frames per second (FPS). Optimal imaging rate depends on exact application, and may be faster or slower than 30 FPS. EM-CCD cameras can typically image up to 60 FPS (full frame) and sCMOS cameras up to 40-100 FPS (full frame).
- Accueil
- Applications
- Sciences de la vie
- Drug Discovery