- fr Change Region
- Global Site
- Accueil
- Ressources
- Notes d'application
Notes d'application
Confocal imaging of immune cells in a vasculature network using the HUMIMIC Chip
novembre 2024
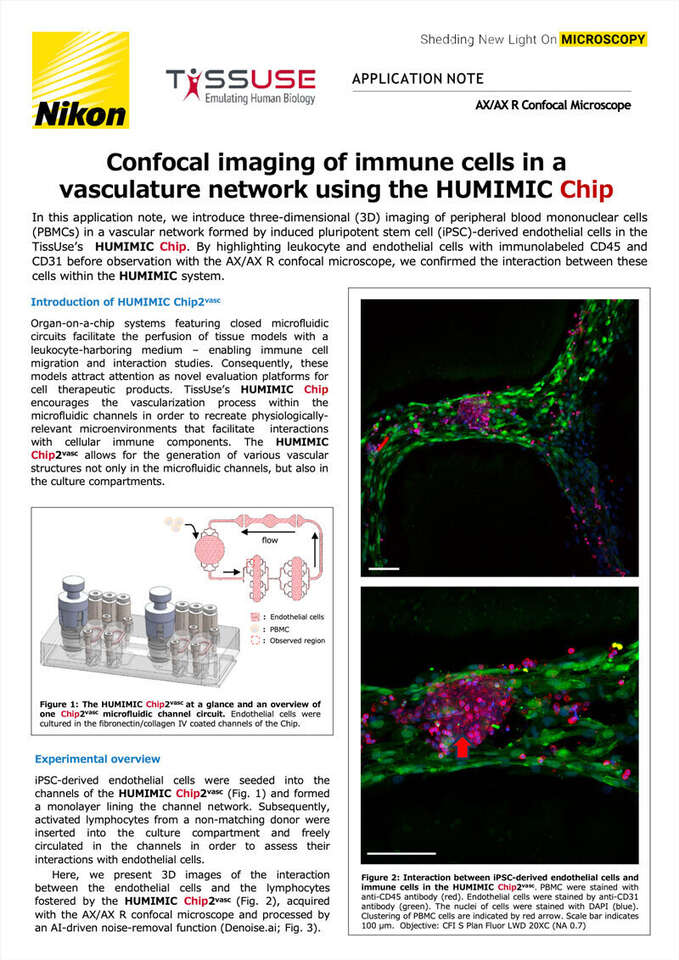
In this application note, we introduce three-dimensional (3D) imaging of peripheral blood mononuclear cells (PBMCs) in a vascular network formed by induced pluripotent stem cell (iPSC)-derived endothelial cells in the TissUse’s HUMIMIC Chip. By highlighting leukocyte and endothelial cells with immunolabeled CD45 and CD31 before observation with the AX/AX R confocal microscope, we confirmed the interaction between these cells within the HUMIMIC system.
Introduction of HUMIMIC Chip2vasc
Figure 1: The HUMIMIC Chip2vasc at a glance and an overview of one Chip2vasc microfluidic channel circuit. Endothelial cells were cultured in the fibronectin/collagen IV coated channels of the Chip.
Organ-on-a-chip systems featuring closed microfluidic circuits facilitate the perfusion of tissue models with a leukocyte-harboring medium – enabling immune cell migration and interaction studies. Consequently, these models attract attention as novel evaluation platforms for cell therapeutic products. TissUse’s HUMIMIC Chip encourages the vascularization process within the microfluidic channels in order to recreate physiologically-relevant microenvironments that facilitate interactions with cellular immune components. The HUMIMIC Chip2vasc allows for the generation of various vascular structures not only in the microfluidic channels, but also in the culture compartments.
Experimental overview
iPSC-derived endothelial cells were seeded into the channels of the HUMIMIC Chip2vasc (Fig. 1) and formed a monolayer lining the channel network. Subsequently, activated lymphocytes from a non-matching donor were inserted into the culture compartment and freely circulated in the channels in order toassess their interactions with endothelial cells.
Here, we present 3D images of the interaction between the endothelial cells and the lymphocytes fostered by the HUMIMIC Chip2vasc (Fig. 2), acquired with the AX/AX R confocal microscope and processed by an AI-driven noise-removal function (Denoise.ai; Fig. 3).
Figure 2: Interaction between iPSC-derived endothelial cells and immune cells in the HUMIMIC Chip2vasc. PBMC were stained with anti-CD45 antibody (red). Endothelial cells were stained by anti-CD31 antibody (green). The nuclei of cells were stained with DAPI (blue). Clustering of PBMC cells are indicated by red arrow. Scale bar indicates 100 μm. Objective: CFI S Plan Fluor LWD 20XC (NA 0.7)
Figure 3: Comparison of images before and after applying Denoise.ai.
PBMC were stained with anti-CD45 antibody (red). Endothelial cells were stained by anti-CD31 antibody (green). The nuclei of cells were stained with DAPI (blue). The Denoise.ai plugin enhances the quality of images by employing a pre-trained network that identifies and eliminates shot noise from confocal datasets. The image on the left represents the original image prior to applying Denoise.ai, while the image on the right depicts the result after utilizing Denoise.ai. PBMC and endothelial cells are well separated in the image after utilizing Denoise.ai (yellow allows). Scale bar indicates 20 μm. Objective: CFI S Plan Fluor LWD 20XC (NA 0.7)
Interaction of PBMCs and endothelial cells using the HUMIMIC Chip2vasc
The images in Figure 2 reveal the clustering of immune cells on the iPSC-derived endothelial cells, resulting in the disruption of the endothelial cell monolayer. Notably, these immune cells were activated by a mixed lymphocyte reaction (MLR) prior to introduction into the chip system. In this process, immune cells from a donor distinct from the endothelial cell donor were pre-activated with CD3-depleted immune cells obtained from the endothelial cell donor, over a span of one week. This interaction of immune cells and endothelial cells serves to establish a dynamic rejection model in the HUMIMIC Chip2vasc.
Summary
Confocal microscopy was employed to observe and analyze the interaction between immune cells and iPSC-derived endothelial cells within the TissUse chip system (HUMIMIC Chip2vasc). The captured images provide valuable insights into these cellular interactions, serving as a foundational tool for subsequent experiments. For instance, in the context of rejection reaction treatment, these images can be utilized to identify and analyze morphological changes between different treatments. As a result, they are anticipated to play a pivotal role in drug discovery screening and the pharmacological evaluation of drug effectiveness, with widespread utilization expected.
AUTHOR INFORMATION
Isabell Durieux1, Hans-Dieter Volk1, Anna-Catharina Krebs1, Anja Hellwig2, Eva-Maria Dehne2, Johanna Dietzfelbinger2, Shingo Nagawa3, Mamiko Masutani3, Makiko Takubo3
1 Berlin Institute of Health (BIH), Charité, Berlin, Germany; 2 TissUse GmbH, Berlin, Germany, www.tissuse.com; 3 NIKON CORPORATION, Tokyo, Japan. This work is supported by EU-H2020 “ReSHAPE” Project, Grant Agreement n. 825392.
Product information
HUMIMIC Starter
Pumping pressure can be continuously adjusted then set automatically by the HUMIMIC Starter. This product is also equipped with a USB-port for easy management of pressure profiles and transfer of data. The HUMIMIC Starter is compatible with HUMIMIC Chip2, Chip3, Chip3plus and Chip4.
HUMIMIC Chip2vasc
The HUMIMIC Chip2vasc is designed for endothelial cells to spread and cover all sides of the microfluidic channels. These channels are connected to a vascular bed where they can sprout to build the vascular connection to the organ models.
These microscopes achieve high resolution images of 8K x 8K pixels, which is four times that of conventional models. A large FOV with a diagonal of 25 mm allows acquisition of a large area of samples in a single scan, reducing phototoxicity. The AX R’s resonant scanner achieves a high resolution of 2K x 2K pixels, allowing acquisition of live sample dynamics with high-speed imaging of up to 720 fps (2048 x 16 pixels).
- Accueil
- Ressources
- Notes d'application
