- en Change Region
- Global Site
Application Notes
High-speed, label-free THG imaging of red blood cells using a multiphoton microscope
January 2024
Multiphoton microscopy can utilize a nonlinear optical phenomenon called harmonic generation to make it possible to observe specific unstained biological materials, in addition to autofluorescence. Second harmonic generation (SHG) occurs when incident light interacts within a material to produce light that is doubled in energy and one half of the original wavelength. Similarly, third harmonic generation (THG) produces light that is one third of the incident wavelength. Because these effects are material-dependent, SHG enables label-free observation of collagen fibers, myosin in striated muscle, etc., while THG allows label-free observation of cell membranes, axons, etc. In this application note, we introduce an example of high-speed, label-free THG imaging of red blood cells using the AX R MP multiphoton confocal microscope.
Keywords: multiphoton microscope, label-free imaging, in vivo imaging
Overview of experiment
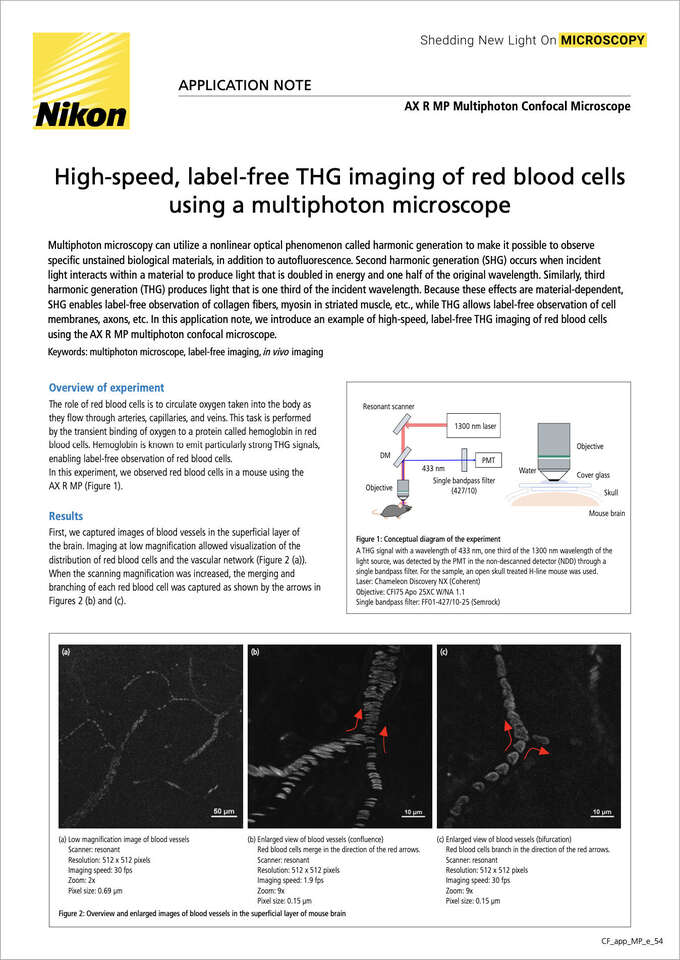
Figure 1: Conceptual diagram of the experiment
A THG signal with a wavelength of 433 nm, one third of the 1300 nm wavelength of the light source, was detected by the PMT in the non-descanned detector (NDD) through a single bandpass filter. For the sample, an open skull treated H-line mouse was used.
Laser: Chameleon Discovery NX (Coherent)
Objective: CFI75 Apo 25XC W/NA 1.1
Single bandpass filter: FF01-427/10-25 (Semrock)
The role of red blood cells is to circulate oxygen taken into the body as they flow through arteries, capillaries, and veins. This task is performed by the transient binding of oxygen to a protein called hemoglobin in red blood cells. Hemoglobin is known to emit particularly strong THG signals, enabling label-free observation of red blood cells.
In this experiment, we observed red blood cells in a mouse using the AX R MP (Figure 1).
Results
First, we captured images of blood vessels in the superficial layer of the brain. Imaging at low magnification allowed visualization of the distribution of red blood cells and the vascular network (Figure 2 (a)). When the scanning magnification was increased, the merging and branching of each red blood cell was captured as shown by the arrows in Figures 2 (b) and (c).
(a) Low magnification image of blood vessels
Scanner: resonant
Resolution: 512 x 512 pixels
Imaging speed: 30 fps
Zoom: 2x
Pixel size: 0.69 µm
(b) Enlarged view of blood vessels (confluence)
Red blood cells merge in the direction of the red arrows.
Scanner: resonant
Resolution: 512 x 512 pixels
Imaging speed: 1.9 fps
Zoom: 9x
Pixel size: 0.15 µm
(c) Enlarged view of blood vessels (bifurcation)
Red blood cells branch in the direction of the red arrows.
Scanner: resonant
Resolution: 512 x 512 pixels
Imaging speed: 30 fps
Zoom: 9x
Pixel size: 0.15 µm
Figure 2: Overview and enlarged images of blood vessels in the superficial layer of mouse brain
Next, we obtained the size and migration speed of red blood cells. To capture the dynamics of red blood cells in real time, we performed high-speed time-lapse imaging at 226 fps using band scanning, which reduces the number of pixels in the Y direction while retaining full-range resonant scanning in the X direction. The center of gravity of red blood cells was tracked (Figure 3 (c)) after denoising by machine learning (Denoise.ai) (Figure 3 (a)) and segmentation (Figure 3 (b)). This revealed that the average size of red blood cells was 7.1 µm (long axis) and the migration speed was 0.18 µm/ms. Since the imaging time per red blood cell is about 1.7 ms, we calculated that the red blood cell has moved about 0.3 µm during this period. Therefore, the image of the red blood cells may have been stretched by about 0.3 µm in the direction of travel compared to the true size of the cell. The potential artifact is only 4% of the long axis of red blood cells, which is a sufficiently small value for analysis of migration speed. This experiment highlights the capability of the resonant scanner for imaging high-speed biological phenomena.
Figure 3: Measurement of migration speed of red blood cells by high-speed imaging
(a) Raw data and image after Denoise.ai processing. The result of the line profile is shown on the right.
(b) Image after segmentation. The blue line indicates the long axis of a red blood cell.
(c) Image after tracking. The yellow dotted line indicates the trajectory connecting the centers of gravity of red blood cells.
[Imaging conditions]
Scanner: resonant
Resolution: 512 x 64 pixels
Imaging speed: 226 fps
Zoom: 9x
Pixel size: 0.15 μm
Number of bits: 12 bits (4096 gradations)
Finally, we evaluated the deformability of red blood cells. Red blood cells are known to change shape when passing through narrow spaces such as capillaries, so we performed quantitative analysis using the time-lapse images shown in Figure 3. Figure 4 (b) shows time-lapse images of red blood cells migrating into the small blood vessel indicated by the yellow arrow in Figure 4 (a). These images captured red blood cells dynamically changing their shape depending on the diameter of the blood vessel. The analysis revealed that the ellipticity of the cell shape changed significantly from 1.4 to 2.4 (Figure 4 (c)).
Figure 4: Measurement of red blood cell deformability
(a) The vessel wall is indicated by the green line, and the directions of red blood cell flow are indicated by the yellow and blue arrows.
(b) Time-lapse images of red blood cells flowing in the direction of the yellow arrow
(c) Measurement results of ellipticity from the time-lapse images. The red arrowheads indicate the ellipticity at each timing in (b).
Summary
Label-free visualization of red blood cells was accomplished via THG imaging using the AX R MP. In addition, the dynamics and shape changes of red blood cells could be captured by high-speed imaging using a resonant scanner.
Lifestyle-related diseases such as arteriosclerosis, diabetes, high blood pressure disorder, and cerebrovascular disease are popular topics of study in recent years. Many in-vivo high-speed phenomena, such as red blood cell flow, can be observed using the method introduced here. We believe that the label-free and high-speed nature of this method will contribute to elucidating pathological conditions and developing treatments.
Acknowledgments
We would like to express our sincere gratitude to Dr. Tomomi Nemoto, head of the Exploratory Research Center on Life and Living Systems at the National Institutes of Natural Sciences (ExCELLS), and Dr. Hirokazu Ishii and Ms. Yuki Watakabe of the Biophotonics Research Group at the same center, for their generous cooperation in preparing, providing, and imaging of samples, and providing biological opinions.
Product Information
AX R MP multiphoton confocal microscope
Realizes a large field-of-view and high-speed, high-resolution deep imaging. Large space for flexible sample settings.
- Large FOV: field number 22
- High speed: up to 720 fps (2048 x16 pixels) for resonant scanning
- High resolution: up to 8K pixels for galvano, 2K pixels for resonant scanning
