- en Change Region
- Global Site
- Home
- Applications
- Life Sciences
- Regenerative Medicine
Regenerative Medicine
Regenerative medicine is the medical field concerned with the development of cell-based therapies for replacing/repairing/treating diseased or injured tissues and organs. Stem cells are principally utilized due to their capacity for differentiation into multiple cell types. Both multipotent stem cells, such as mesenchymal stem cells (MSCs), and pluripotent stem cells, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), are used to manufacture therapeutics.
The reliable culture, differentiation, and application of stem cells is thus of great importance in regenerative medicine. “Cell manufacturing” of stem cells at scale is most reliably performed using a light microscope to quantitatively determine and assess key indicators related to culture state and quality. Such indicators may include proliferation rate, colony formation characteristics, morphological features, etc.
Products for Regenerative Medicine
The Nikon ECLIPSE Ts2 and Ts2-FL inverted microscopes are award-winning compact inverted microscopes designed for routine cell culture-related work. The Ts2-FL has all the features of the Ts2 but additionally provides built-in LED-based epifluorescence. These microscopes are the usual choice for more routine cell culture work and used more widely in basic research than manufacturing.
●: included, ⚬: option
| ECLIPSE Ts2 Inverted Microscopes |
|
|---|---|
| Diascopic Illumination Source | Blue LED-excited white phosphor |
| Position Control | Manual |
| Camera Integration | Free Selection (Optional) |
| Accommodates well plates, dishes, and flasks | yes |
| Compatible with decontamination using vaporized H2O2 | no |
| Suitable for cell manufacturing facilities | no |
| Compatible Contrasting Techniques | ECLIPSE Ts2 |
| Brightfield | yes |
| Widefield Fluorescence | TS2-FL |
| Nikon Emboss Contrast | yes |
| Phase Contrast | yes |
Related Literature
Discussion of Regenerative Medicine
From Cell Culture to Cell Manufacturing


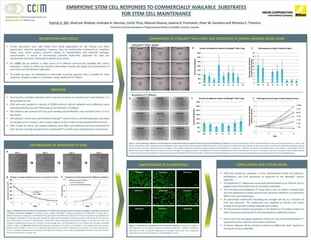
Application of the Cell Analysis Module to create a mask image of iPSC colonies present in phase contrast data, allowing for measurement of the number of colonies and their respective area.
Cell culture/manufacturing to produce a therapeutic product is very different than cell culture as routinely performed in an academic or basic research setting. Manufacturing in the healthcare and pharmaceutical industries requires a greater level of quality control and operational efficiency and is subject to significantly more regulation. Predictable production of a quality product that meets the required standards while minimizing cost is the ideal.
The application of image analysis to inform decision-making helps remove one of the greatest barriers towards predictable manufacturing – the reliance on human operators, which inherently introduces variability, even with an experienced staff. Qualitative methods, e.g., “shaking the vessel three times after seeding” can be put to the test using quantitative image analysis techniques and thus formalized.
Application of engineered 3D stem cell-derived systems for patient implantation is already happening, e.g., the culture of skin grafts. Clinical testing of more complicated cell systems is underway. The eventual goal is to be able to culture fully functional complex organs such as the heart. Note that 3D engineered tissues, including various types of organoids, are also applied towards drug discovery testing. Click to see our Drug Discovery Solutions page for more information concerning this application.
Minimizing Perturbation to Sensitive Cell Cultures
A variety of cell types are cultured in vitro, including primary cells recently obtained from tissue, immortalized cell lines, and stem cells. Cultures of immortalized cell lines, as often used for basic biological research, can be quite capable of withstanding significant insult, such as improper seeding density, being left outside of the incubator for extended periods, etc. Stem cells tend to be more sensitive to perturbation. Stringent attention to culture conditions is thus important when working with stem cells, especially when cultured for use as part of a therapeutic.
Phototoxicity is also a concern. For this reason, a low illumination intensity technique such as phase contrast is usually favored. Fluorescence labeling can be an invasive procedure and fluorescence imaging can be phototoxic.
Cell x Image Lab – A Resource for Cell Culture Success
Supporting the regenerative medicine field is a strategic goal of Nikon Healthcare. To this end, we have launched the Cell x Image Lab website, which details how microscopy imaging and analysis can be used to give cell culture processes and procedures a quantitative edge – enhancing the success rate and reproducibility of cell manufacturing. Imaging and analysis are also useful for quality assurance and developing new processes and procedures.
The Cell x Image Lab website includes both an introductory section with basic information about the field as well as numerous case studies detailing specific imaging and analysis applications for cell culture and manufacturing.
Glossary
- Accommodates well plates, dishes, and flasks
- These are the common types of vessels used for culturing cells. This category refers to the availability of Nikon stages/inserts for standard size vessels.
- Camera Integration
- Nikon provides a variety of camera options, including fast color and monochrome CMOS options.
- Compatible Contrasting Techniques
- Phase contrast is the standard for cell culture and manufacturing, however sometimes other techniques (e.g., fluorescence) are required.
- Compatible with decontamination using vaporized H2O2
- Vaporized hydrogen peroxide decontamination is an industry standard for enclosed spaces. It is widely applied in hospitals and other medical facilities.
- Diascopic Illumination Source
- Illumination sources for diascopic transmitted light-based contrast techniques are usually either light emitting diodes (LEDs) or halogen lamps. LEDs are a bright, eco-friendly, and more energy efficient choice.
- Position Control
- The ability to translate the sample or scan position using motorized system components, for example, using a motorized Z drive to move the objective lens and corresponding focal plane.
- Suitable for cell manufacturing facilities
- This refers to whether systems can perform common types of assays applied in cell manufacturing facilities (e.g., stem cell colony identification).
- Home
- Applications
- Life Sciences
- Regenerative Medicine