- ko Change Region
- Global Site
Application Notes
3D Confocal Imaging of Thick Samples Using Z Intensity Correction
2024 년 10 월
Confocal microscopy is a technique that captures clear, cross-sectional images by filtering out light that isn‘t in focus. By continuously capturing images along the optical axis (Z-axis), it is possible to obtain high-definition, high-resolution 3D images. However, in conducting 3D imaging of thick samples, light is increasingly attenuated due to refraction and scattering as the focal plane is moved further into the sample. This results in uneven brightness between successive images in the Z direction. The NIS-Elements imaging software is equipped with a function called ‘Z Intensity Correction’, which can be used to acquire thick sample z-stack images with uniform brightness by adjusting the Laser Power and Gain according to the Z position.
Microphysiological Systems (MPS) have recently been attracting attention as a novel approach in nonclinical drug testing for pharmaceutical research, and often require 3D imaging to be fully observed. In this application note, we examine a human proximal tubule model constructed within a collagen gel using a microfluidic MPS chip and assess the effectiveness of Z Intensity Correction towards 3D imaging.
Effectiveness of Z Intensity Correction
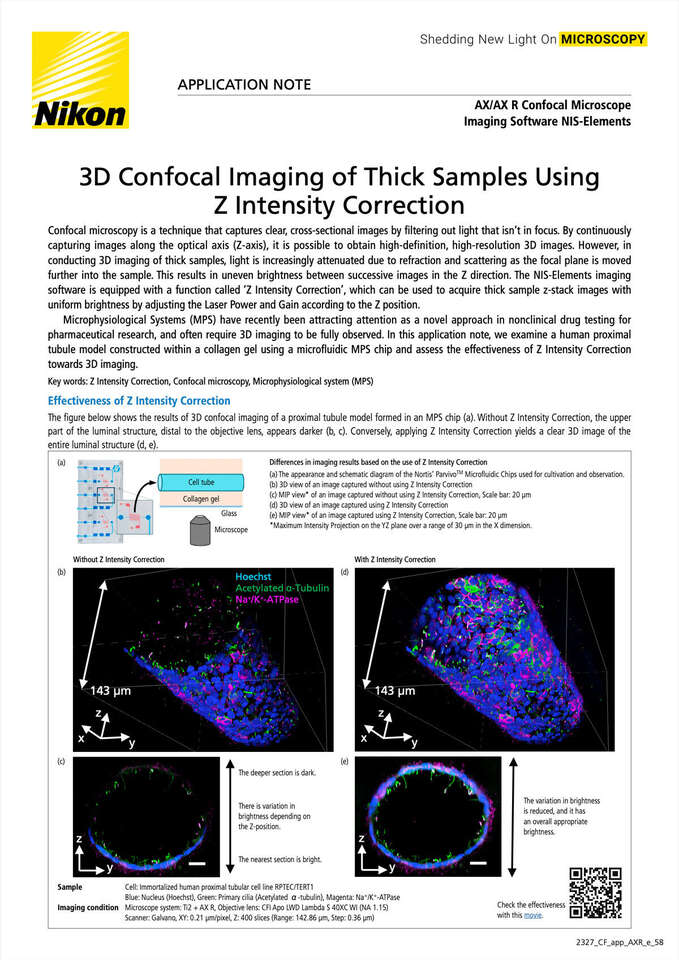
The figure below shows the results of 3D confocal imaging of a proximal tubule model formed in an MPS chip (a). Without Z Intensity Correction, the upper part of the luminal structure, distal to the objective lens, appears darker (b, c). Conversely, applying Z Intensity Correction yields a clear 3D image of the entire luminal structure (d, e).
Differences in imaging results based on the use of Z Intensity Correction
(a) The appearance and schematic diagram of the Nortis’ ParvivoTM Microfluidic Chips used for cultivation and observation.
(b) 3D view of an image captured without using Z Intensity Correction
(c) MIP view* of an image captured without using Z Intensity Correction, Scale bar: 20 μm
(d) 3D view of an image captured using Z Intensity Correction
(e) MIP view* of an image captured using Z Intensity Correction, Scale bar: 20 μm
*Maximum Intensity Projection on the YZ plane over a range of 30 μm in the X dimension.
Sample
Cell: Immortalized human proximal tubular cell lineRPTEC/TERT1
Blue: Nucleus (Hoechst), Green: Primary cilia (Acetylated α-tubulin), Magenta: Na+/K+-ATPase
Imaging condition
Microscope system: ECLIPSE Ti2 + AX R, Objective lens:CFI Apo LWD Lambda S 40XC WI (NA 1.15)
Scanner: Galvano, XY: 0.21 μm/pixel, Z: 400 slices (Range: 142.86 μm, Step: 0.36 μm)
What is Z Intensity Correction?
In conventional 3D imaging, the settings for Laser Power and Gain remain constant regardless of the focal plane Z-positions. This can often cause samples to appear darker at greater depths due to factors specific to the sample, like refraction and scattering. Z Intensity Correction works to createuniformly bright 3D imaging from top to bottom by gradually adjusting Laser Power and Gain based on the Z-position.
The figure on the right shows an example of 3D imaging captured from Z=1 to Z=100. When using the constantLaser Power and Gain settings regardless of the Z-position, the image becomes darker as the Z value increases (as light from the focal plane passes through more sample material before reaching the objective lens). When registering appropriate settings for Z=1, 40, and 100 in Z Intensity Correction, a linearly interpolated setting function is applied in the Z direction during 3D imaging. This results in a 3D image with uniform brightness in the Z-direction.
How to use Z Intensity Correction
- Right-click on the background of NIS-Elements and open the menu. Select Acquisition Controls > Z Intensity Correction to open the control panel (Operation panel 1).
- While viewing the actual sectional image that you want to use as a brightness reference, adjust the Laser Power and Gain to suitable settings for imaging.
- Click the [+] button to register the Z position and the adjusted Laser Power and Gain (Operation panel 2).
- Move the Z position and adjust the Laser Power and Gain to match the brightness set in step 2 and 3. Register the settings again by clicking the [+] button.
- Repeat step 4 at various Z positions until the entire imaging range is properly adjusted.
- Click the [Run Z Corr] button in ND Acquisition panel to execute (Operation panel 3).
✔︎ The range of Z for imaging should be set in ND Acquisition panel.
Summary
With Z intensity Correction, the luminal structure of the proximal tubule model in 3D collagen matrix can be clearly captured, from top to bottom. Z Intensity Correction is applicable towards 3D imaging of a wide variety of thick samples.
Product Information
NIS-Elements Imaging Software
Z Intensity Correction
Unlock the full potential of your confocal microscope with Z Intensity Correction. Ensure consistent brightness and reveal intricate details when imaging thick samples across a wide range of Z positions.
Compatible software package: NIS-Elements AR, C, C-ER
AX/AX R Confocal Microscope
Supports high-speed, high-resolution, wide-field confocal imaging with low phototoxicity to living cells and minimal photobleaching.
- High speed: Fastest 720 frames per second
(Resonant 2048 x 16 pixel) - High resolution: up to8K (Galvano scanner)
/ 2K (Resonant) - High throughput: Ultra wide field of view (25 mm)
