- es Change Region
- Global Site
Notas de aplicación

Analysis of nuclear translocation using AI-driven fully automated ECLIPSE Ji
septiembre 2023
ECLIPSE Ji with Smart Experiment software enables seamless experiments from image acquisition to analysis and graph creation. Pre-trained Artificial Intelligence (AI) and pre-defined imaging processes automatically optimize image acquisition and analysis settings, providing visualized data and EC50 information with simple operation. The transcription factor NF-κB is widely known as a drug target for cancer and inflammatory diseases. Activated NF-κB translocates from the cytoplasm to the nucleus and induces the expression of disease-related genes. This application note introduces an example of using the Nuclear Translocation module of Smart Experiment to quantify the TNF-α-stimulated NF-κB translocation from the cytoplasm to the nucleus.
Keywords: nuclear translocation, signaling pathway, anticancer drug, anti-inflammatory, automatic setting, EC50, dose-response curve
Experimental overview
(1) HeLa cells were seeded in 96-well plates and cultured for 24 hours. (2) The test substance TNF-α was diluted to 10 different concentrations and added to each well and treated for 30 minutes. (3) Cells were fixed with 4% PFA. Membrane permeabilization was induced with 0.1% Triton X-100. (4) Immunofluorescence staining included Rabbit anti NF-κB p65 antibody and Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488). Nuclei were stained with DAPI. (5) The well plate was placed on ECLIPSE Ji and image acquisition and analysis was run automatically by selecting the Nuclear Translocation analysis icon.
| Detection region | Fluorescence label | Ex/Em (nm) |
|---|---|---|
| Nucleus region | DAPI | 345/455 |
| Target protein | Rabbit anti NF-KB p65 antibody (primary), Goat Anti-Rabbit IgG H&L (Alexa Fluor@ 488) (secondary) | 495/519 |
| Magnification | Field of view | |
| 20X | 0.88 × 0.88 mm | |
Table. 1: Detection regions, fluorescence labels, and image acquisition conditions
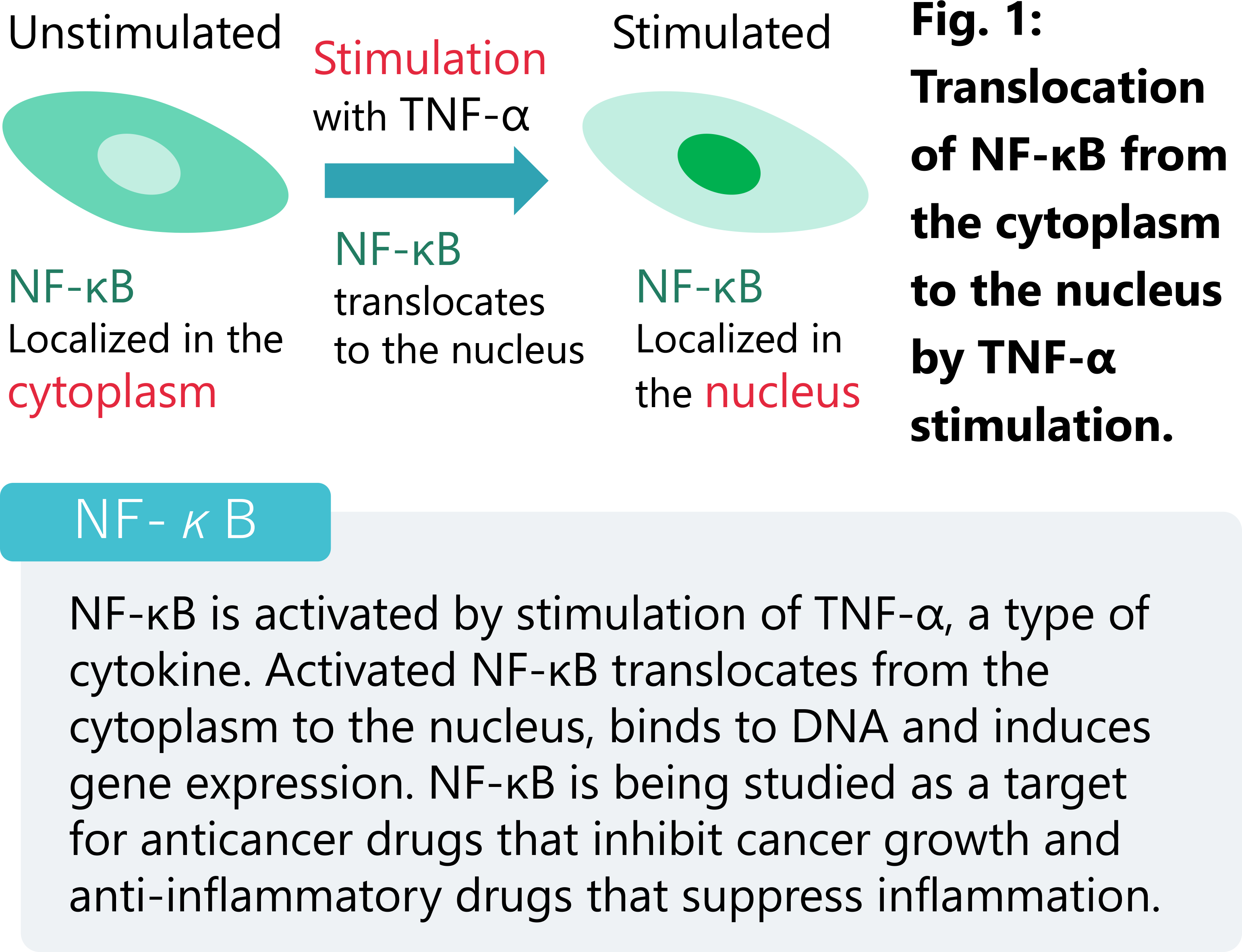
Fig. 2: Definition of mask region and quantification method of nuclear translocation.Upper row: A nuclei mask (yellow) is created from the inside of the nucleus region detected by DAPI.A ring-shaped mask (green) outside the nucleus defines the cytoplasmic region. Nuclear translocation of NF-κB is quantified by measuring the fluorescence (FL) intensity ratio of the inner nucleus (yellow) and the cytoplasm ring (green). Middle row: Control image (NF-κB localized in the cytoplasm), Lower row: Image treated with TNF-α 200 ng/mL for 30 minutes (NF-κB localized in the nucleus). Immunofluorescence staining images of NF-κB (green) in HeLa cells.
Fig. 3: Images and dose-response curves of HeLa cells treated with control and TNF-α for 30 min.
(A, B) Immunofluorescence staining images of NF-κB (green) in HeLa cells, scale bar: 100 μm, (A) control, (B) Image treated with TNF-α 200 ng/mL for 30 minutes, (C) Dose-response curve showing the results of TNF-α dose-dependent increase in nuclear translocation of NF-κB, X-axis: drug concentration (log), Y-axis: fluorescence intensity ratio of nucleus and cytoplasm (average), NucCytoRatio: Nuclei Circle Fluorescence intensity/Cytoplasm Ring Fluorescence intensity, EC50 = 0.950ng/mL、Z’-factor = 0.724, Left image: NF-κB is localized in the cytoplasm. Right image: NF-κB is localized in the nucleus.
Summary
- A nuclear translocation module allowed us to quantify nuclear translocation of NF-κB in a dose-dependent increases in TNF-α. The EC50M/sub> was calculated to be 0.950 ng/mL.
- The ratio of fluorescence intensity between nucleus and cytoplasm regions allows quantification of protein nuclear translocation and calculation of EC50 for cytokines and drugs.
- Smart Experiment can be run automatically from image acquisition to analysis and graph creation.
- A pretrained "CellFinder.ai" finds the optimal focal plane, there is no need to set tedious autofocus.
- Image acquisition and analysis conditions are predefined and no advanced imaging knowledge is required. Anyone can easily obtain analysis results.
- It is a simple operation to place the well plate on Ji, select the Nuclear Translocation analysis icon, and input the sample information. Under the conditions of this experiment, the automated routine took approximately 17 minutes from the start of imaging to graph display.
- Researchers can concentrate on more creative research activities by leaving tedious tasks to AI.
Sample preparation protocol
- HeLa cells are seeded in a 96-well plate at a density of 1x10e4 cells/well and culture for 24 hours at 37℃ in a 5% CO2 incubator.
- TNF-α is diluted with medium to concentrations of 0, 0.03, 0.09, 0.27, 0.82, 2.47, 7.40, 22.2, 66.7 and 200 ng/mL, and each concentration of medium containing 0.1% BSA is added to each of the 6 wells. Cells are treated with TNF-α for 30 minutes at 37°C in a 5% CO2 incubator.
- Wash cells once with PBS.
- 4% PFA is added to the wells and leave at room temperature for 10 minutes to fix the cells.
- Wash cells 3 times with PBS.
- Add 0.1% Triton X-100, 0.1% BSA in PBS to the wells and leave at room temperature for 5 minutes for membrane permeabilization.
- Wash cells 3 times with PBS.
- Add the primary antibody (1:200) diluted with 0.1% BSA in PBS to the wells and leave at room temperature for 1 hour.
- Wash cells 3 times with PBS.
- Add the secondary antibody (1:500) diluted with 0.1% BSA in PBS to the wells and leave at room temperature for 1 hour.
- Wash cells 3 times with PBS.
- DAPI (2 μg/mL) is added to the wells and leave at room temperature for 5 minutes.
- Wash cells 3 times with PBS.
Materials and reagents
Cell Culture
| Cell Line | HeLa (RIKEN RCB0007) |
|---|---|
| Growth medium | MEM + 10%FBS + 1%Pc/Sm |
| Culture vessel | EZVIEW ® Culture Plate B (Glass Bottom Plate) Microplate 96 well (AGC techno glass (IWAKI), 5866-096) |
Test substance
| Substance | TNF-α |
|---|---|
| Test concentration | Negative control: 0 ng/ml |
| Plate map example | 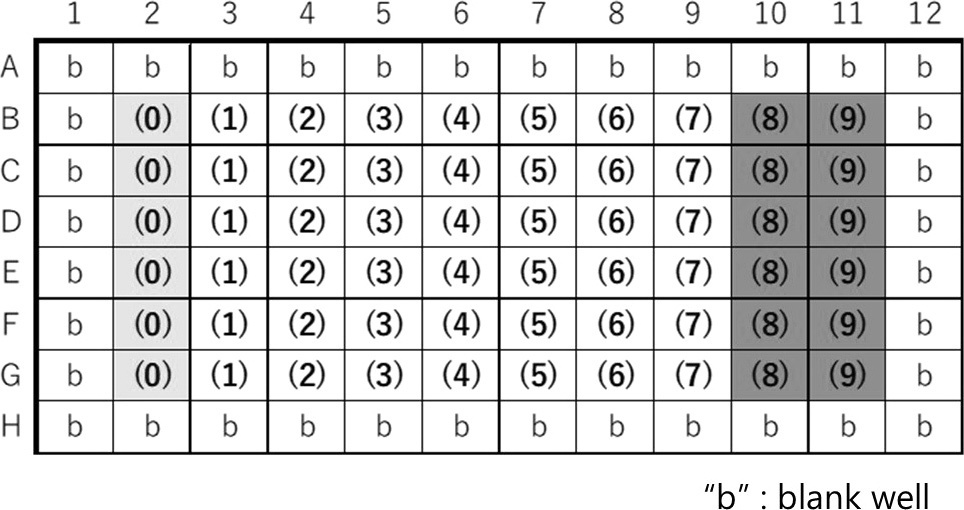 |
Reagents
| Product name | Item number | Supplier |
|---|---|---|
| Recombinant Human TNF-α | 300-01-A | PeproTech |
| Rabbit anti NF-κB p65 antibody (primary antibody) | ab16502 | Abcam |
| Goat Anti-Rabbit IgG H&L (Alexa Fluor ® 488) (secondary antibody) |
ab150077 | Abcam |
| DAPI Solution | 62248 | Thermo Fisher Scientific |
| MEM (Minimum Essential Medium) | 11095080 | Thermo Fisher Scientific |
| Fetal bovine serum (FBS) | 10437028 | Thermo Fisher Scientific |
| Penicillin-Streptomycin (Pc/Sm) (10,000 U/ml) |
15140122 | Thermo Fisher Scientific |
| 16%-Paraformaldehyde Aqueous Solution (16% PFA) *Dilute by 4% with PBS before use |
11850-14 | Nacalai Tesque |
Compatible vessel*
- 24-well plate
- 96-well plate
*Compatible with only glass bottom well plates.
Reference
Trask, OJ, Nuclear Factor Kappa B (NF-κB) Translocation Assay Development and Validation for High Content Screening. Assay Guidance Manual (2012)
Product information
Smart Imaging System ECLIPSE Ji
ECLIPSE Ji is an AI-Driven, fully automated imaging system. By using it in combination with NIS-Elements SE, image acquisition, analysis, and graph creation can be run seamlessly and automatically. It is equipped with "CellFinder.ai“, which uses AI to find the optimal focal plane in autofocus settings that normally require advanced human judgment. Various trained AIs are implemented in the image acquisition and analysis process. This greatly reduces the number of steps for setting and optimization and makes it easier for everyone to get results.
Imaging Software NIS-Elements SE Nuclear Translocation (Option)
- Fully automated from image acquisition to analysis and graph display.
- Protein translocation from the cytoplasm to the nucleus can be easily analyzed in an automated manner.
- One-click reports can be created and output with PDF including images, analysis results, dose-response curves, and EC50/IC50 calculation results.
- Cellular imaging and analysis with Ji is easier and more comfortable.
