- fr Change Region
- Global Site
- Accueil
- Ressources
- Notes d'application
Notes d'application

Episcopic brightfield imaging of neuronal cells on high-density microelectrode arrays enables prediction of cell region through AI learning
décembre 2023
High-density microelectrode arrays (MaxOne and MaxTwo MultiwellHD-MEAs, MaxWell Biosystems) are platforms for electrophysiology research that enable simultaneous recording and stimulation of electrogenic cells such as retinal ganglion cells, cardiomyocytes, human induced pluripotent stem cell (iPSC)-derived cells, brain slices, and brain organoids. These models have extensive applications in neuroscience research, drug discovery, cell analysis and so on. However, these arrays block transmitted light and prevent the use of label-free live cell imaging techniques such as phase contrast microscopy. Therefore, it is necessary to fix the cells and perform immuno- or dye staining to observe them. This application note introduces a case study wherein human iPSC-derived neuronal cells were seeded on the arrays and imaged with the ECLIPSE LV100ND upright microscope, enabling the assessment of neuronal cell attachment to the array through analysis utilizing the AI technology of NIS-Elements software.
Keywords: MaxOne, MaxTwo, high-density microelectrode array, human iPSC-derived neurons, episcopic brightfield observation, AI
Experiments
1. Cell culture
■ Cells
- Quick-Neuron™Excitatory-Human iPSC-derived Neurons (Healthy Control) (Elixirgen Scientific, EX-SeV-HC, Lot:QNGSVF-CW50065-20190131T-1M)
■ Reagent
- Poly-L-ornithine solution (Sigma-Aldrich, P4957-50ML)
- DMEM/F-12, no glutamine (Thermo Fisher Scientific, 21331-020)
- Neurobasal™ Medium (Thermo Fisher Scientific, 21103049)
- GlutaMAX™ Supplement (Thermo Fisher Scientific, 35050061)
- Penicillin-Streptomycin, Liquid (Thermo Fisher Scientific, 15140-122)
- Laminin Mouse Protein, Natural (Thermo Fisher Scientific, 23017015)
- Y-27632 2HCl (Selleck Chemicals, s1049)
- Quick-Neuron™ Excitatory -Maintenance Medium- (Elixirgen Scientific, EX-MM)
- Cellstain®- Calcein-AM solution (DOJINDO, C396)
- MaxOne HD-MEA Chips (MaxWell Biosystems AG)
■ Method
A MaxOne Chip (Fig. 1a) was coated with 30 μl of Poly-L-Ornithine (0.002%) for 2 hrs, followed by 100 μl of laminin (20 μg/ml) for 2 hrs. Cells were seeded at a density of 2x105 cells per chip and cultured at 37℃ in 5% CO2, according to the Elixirgen Scientific protocol. The culture medium was replaced every 2 days, and 10-day cultured cells were stained with Calcein-AM for live-cell observation.
2. Image acquisition
Eleven episcopic brightfield and calcein fluorescence images covering the same field of view (FOV) of cells loaded on two MaxOne chips (Fig. 2a) were acquired using the LV100ND upright microscope, a TU Plan Fluor EPI 10X objective, and Hamamatsu Photonics ORCA-Flash4.0 camera (16 bit) (Fig. 1b). The episcopic brightfield images were illuminated using 525 nm LED light (EXCELITAS X-Cite Turbo) via a small aperture. The calcein fluorescence images were obtained using 475 nm LED light and a FITC filter cube.
Fig. 1: Episcopic brightfield imaging of live cells on MaxOne chip
(a) Acquisition area on MaxOne Chips
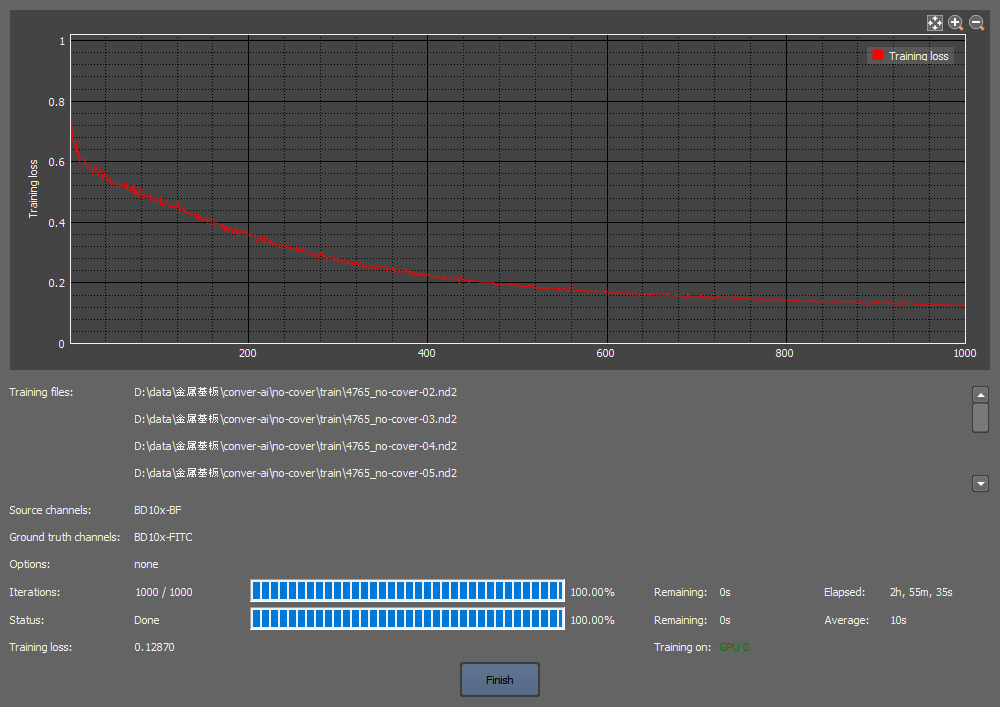
(b) Learning curve
(c) Episcopic brightfield image
(d) AI-predicted image
(e) Calcein fluorescence image
Fig. 2: Estimation of cell region by AI learning model
3. Prediction of cell region using AI learning model
Ten images (Fig. 2a) were used to train an AI learning model by utilizing the episcopic brightfield images as input images and calcein fluorescence images as ground truth images for 1000 iterations through the Convert.ai function of the NIS-Elements AR software. The learning curve of Fig. 2b shows that sufficient learning had been accomplished (training loss 0.129). By analyzing a separate episcopic brightfield image not used for training to the above AI learning model (Fig.2c), the cell region was predicted (Fig. 2d) and compared with the true calcein fluorescence image (Fig. 2e), revealing a close match. [To perform a quantitative assessment, the cell count was determined by processing both the calcein image and the AI-predicted image. The AI predicted 73% of the 2067 calcein-stained cells,corresponding to 80% of the 1886 cells predicted by the AI.]
Summary
Depending on the type of cell culture vessel such as a MaxOne chip, traditional transmitted light microscopy may not be able to evaluate unstained cells. However, the combination of episcopic brightfield microscopy and AI models can improve the reproducibility and reliability of test results by noninvasively observing the seeding density and variability of viable cells on the chip prior to testing. This technology can considerably reduce the number of evaluation samples required to find culture conditions and improve operational effectiveness.
Product Information
ECLIPSE LV100ND upright microscope (Episcopic/diascopicillumination model)
Supports brightfield, fluorescence, DIC, and polarizing observation using episcopic illuminator with a high-intensity halogen lamp, in addition to bright-field, phase contrast, DIC, and polarizing observation using a diascopic illuminator.
TU Plan Fluor EPI 10X objective
This CFI60-2 objective, with a high NA (0.3) and long working distance (17.5mm), is optimized for episcopic observation. Sharp, high-contrast images can be obtained with excellent chromatic aberration correction.
- Accueil
- Ressources
- Notes d'application
