Masato Nakagawa
So-called iPS cells are attracting considerable interest as pluripotent stem cells that may open up a whole new world of medicine. The Center for iPS Cell Research and Application (CiRA) at Kyoto University is pursuing a wide range of research activities that aim to realize regenerative medicine utilizing iPS cells. The Nikon BioStation CT cell culture observation system is being used in this iPS cell research and is contributing to its efficiency.
We were pleased to have had an opportunity to speak with Masato Nakagawa, who is engaged in iPS cell research at CiRA.
Equipo Nikon
CiRA, specializing in a wide range of iPS cell research
Please tell us about CiRA.
CiRA was established in April 2010 as the world's first research institution that specializes in iPS cells. Its mission is to contribute to the realization of regenerative medicine by pursuing the possibilities of iPS cells through both fundamental and applied research. Another important mission of CiRA is to cultivate promising young researchers and promote research collaborations through its close ties with Kyoto University's other institutions, such as the Institute for Frontier Medical Sciences and the University Hospital.

Center for iPS Cell Research and Application, Kyoto University (Image: courtesy of Center for iPS Cell Research and Application, Kyoto University)
Please tell us about the department you belong to.
CiRA is headed by Professor Shinya Yamanaka, who discovered iPS cells, and is divided into five research departments. I belong to the Department of Reprogramming Science. "Reprogramming" refers to the phenomenon whereby somatic (body) cells that have already been specialized as cells of specific tissues or organs return to the pluripotent stem cells they were prior to specialization. Professor Yamanaka succeeded in artificially inducing this reprogramming and gave the name "iPS cell" to this pluripotent stem cell. iPS cells are versatile cells that specialize to become cells of various types of tissues or organs. They can multiply almost without limit. The name "iPS cell," by the way, is an abbreviation of "induced pluripotent stem cell."
At the Department of Reprogramming Science, we are working on clarifying the reprogramming mechanism at the genetic level and developing technologies for producing iPS cells that can be used in regenerative medicine.
Aiming to produce safer iPS cells
How are iPS cells made?
In the case of human skin, for example, when we surgically remove skin and culture it for a month, we get cells that are original iPS cells. Now we can also produce iPS cells from blood cells, and this approach has recently become mainstream. The stress on the body to obtain blood cells is minimal compared to surgically removing skin cells. When we induce these cells with a reprogramming factor, they become iPS cells in about a month and form aggregates of cells called "colonies." One colony comprises several thousand iPS cells.
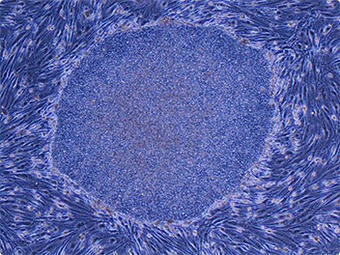
iPS cells derived from adult human dermal fibroblasts (Image: courtesy of Shinya Yamanaka, Center for iPS Cell Research and Application, Kyoto University)
What does "reprogramming factor" mean?
It refers to inducing a gene to reprogram cells to their original state. Professor Yamanaka discovered that four genes—Oct3/4, Sox2, Klf4, and c-Myc—can be used as factors to induce reprogramming. Reproducibility through methods that use these factors is high and it's a real breakthrough as reproduction is relatively easy to perform.
Please tell us more about what your research group does.
As our research progressed, we discovered that c-Myc, one of the four genes, included a factor that induced cancerous changes. While it was also possible to establish iPS cells without using c-Myc, production efficiency was very low and quality was poor as well.
To address these issues, we looked for an alternative for the c-Myc factor. Many experiments later, my research team discovered that we could prevent the risk of cancerous changes if we used L-Myc, which is a relative of c-Myc. We were also able to improve production efficiency by 5-10 times compared to c-Myc.
One of the important aims of my research group is to efficiently produce safer iPS cells.
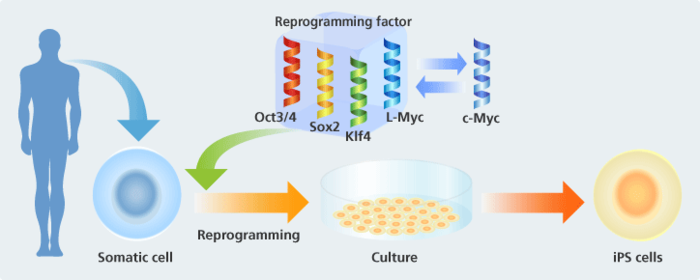
PS/non-iPS cell colony auto identification program contributing to efficiency in iPS cell research
How do you confirm that iPS cells have been produced?
Generally, as many as 150 colonies of iPS cells are produced for each 10-cm culture vessel. The feature of an iPS cell colony, when observed through a microscope, is its flat appearance and clear border. In the past, iPS cell colonies were distinguished visually from colonies of other cells and counted individually. This not only took time but continuously looking into the microscope was physically demanding. Some people even chose to take medicine in order not to become dizzy while counting the colonies. Now, though, we use the Nikon BioStation CT, which has boosted efficiency.
When did you install the BioStation CT?
We installed it in 2008, before CiRA was established. We co-developed an optional program for the BioStation CT with Nikon that automatically identifies colonies of iPS cells and counts them. We did this not just because doing this work took time and was very demanding but because there was also the issue of data reliability when the work was dependent on human effort alone. After the iPS/non-iPS cell colony auto identification program was completed, we rarely needed to make visual counts.
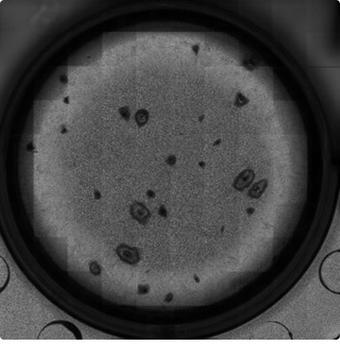
Image captured by the BioStation CT (magnification: 2×) (Image: courtesy of Tatsuya Yamakawa, CiRA, Kyoto University)
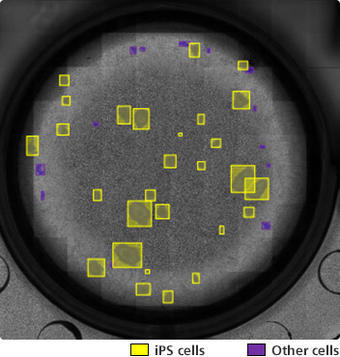
Image of iPS cells automatically distinguished from other cells using the iPS/non-iPS cell colony auto identification program
(Image: courtesy of Tatsuya Yamakawa, CiRA, Kyoto University)
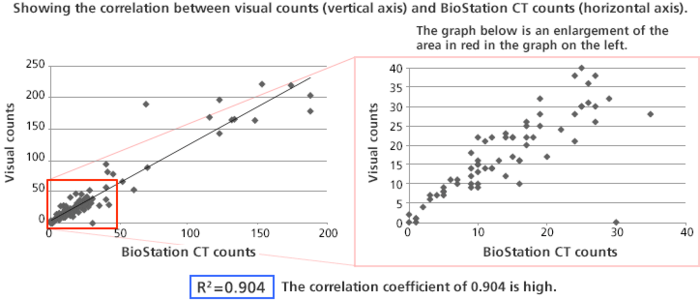
Did you have any difficulties developing the iPS/non-iPS cell colony auto identification program?
We gave Nikon a photo image of colonies with markers indicating iPS cell colonies and non-iPS cell colonies, and had Nikon develop the program. At the beginning, however, it took some time for us to get Nikon to understand the shapes of iPS cells that were "good" cells. There was a lot of difficulty getting these counts to match the visual counts, and each time we had to make a new image with markers and give it to Nikon. It was quite an effort to get Nikon to understand colonies and improve accuracy, but in the end, Nikon was able to meet our needs as well as other requirements concerning hardware. We are very satisfied with the results.

Masato Nakagawa and Tatsuya Yamakawa, a researcher who uses the BioStation CT. As the person in charge, Yamakawa repeatedly verified the iPS/non-iPS cell colony auto identification program and was an important contributor to its development and improvement.
How is your research going now that you are actually using the BioStation CT?
Thanks to the iPS/non-iPS cell colony auto identification program, we are now able to take photo images of up to 30 culture vessels at one time and count the colonies. With the BioStation CT, we can automatically and remotely observe culture vessels without removing them from the incubator, which is a plus for both researchers and cells.
Research efficiency has improved dramatically since we got the BioStation CT. We've identified as many as 2,000 gene types as candidates for reprogramming factors, and we have also produced iPS cells. I can't imagine how we could have done all this without the BioStation CT.
Counting colonies using the BioStation CT
(Source: Tatsuya Yamakawa, CiRA, Kyoto University)
A future of expanding possibilities for iPS cells
Please tell us about the possibilities of iPS cells and their applications in regenerative medicine.
Regenerative medicine is a method of treatment that restores functions that have been lost due to disease or injury. For example, if a nerve has been cut due to an external injury, iPS cells made from the patient's somatic cells can be used to make nerve cells that will reconnect the damaged network. This may be possible through transplant surgery.
For untreatable diseases as well, iPS cells may help in finding the causes of these diseases. In diseases caused by mutations of nerve cells in the brain, for instance, external access may be difficult and even if we can observe the cells that have mutated, it is sometimes difficult to understand how the cells were in their normal state. The use of iPS cells made from the patient's somatic cells has the potential to advance research into these kinds of diseases. We also expect to see great strides made in the development of new drugs.
iPS cell technology was developed in Japan. I would very much like to see Japan's academic and corporate worlds work closely together so that medical technologies utilizing iPS cells can become a viable industry and be of benefit to patients as soon as possible.
Finally, could you say a few words to people who are aiming to become researchers and give us your thoughts on iPS cells?
A huge amount of fundamental research is necessary before the technology can be applied in a practical sense. Through fundamental research, I hope to learn about the truths inherent in cells. This will lead to groundbreaking applications and greater happiness for society. Research is extremely worthwhile work. I very much encourage anyone interested to follow the path of research and make at least one new discovery.
Also, I have a lot of respect for medical doctors, as they can heal injuries and cure diseases, bringing comfort to people in the process. I don't have the qualities necessary to become a doctor, but I'm hoping that I can contribute in some way through my research in iPS cells.

