Dr. Tadahiro Iimura
Division of Bio-Imaging, Proteo-Science Center (PROS), Ehime University
Division of Analytical Bio-Medicine, Advanced Research Support Center (ADRES), Ehime University
Graduate School of Medicine, Ehime University
Equipo Nikon
- N-SIM Super Resolution
- A1+ Confocal (see current model)
Please tell us about your research.
I’m researching bone metabolism at the Division of Bio-Imaging, Proteo-Science Center (PROS), Ehime University, analyzing the pathogenic mechanisms of osteoporosis (an illness we need to overcome in our aging society) and the pharmacological effects of medicine for it using molecular biological approaches and bio-imaging methods. At the Division of Analytical Bio-Medicine, Advanced Research Support Center (ADRES), Ehime University, I lead the management of shared equipment and the commissioned research analysis center in our medical school, in collaboration with our excellent technical members.
What applications do you use Nikon products for?
In the Division of Bio-Imaging at PROS, we use the N-SIM Super Resolution Microscope.
Protein science professionals at PROS actively utilize the N-SIM to acquire spacio-temporal information on the intracellular dynamics of newly-found proteins.
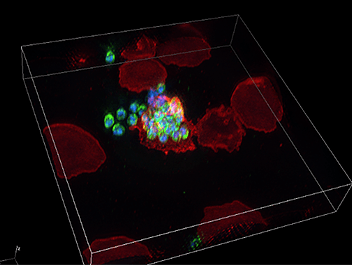
PROS members are open to interdivisional collaborative researches, combining their skilled research methods and expertise. Conducting collaborative research with the Division of Malaria Research at PROS (lead by Prof. Takafumi Tsuboi), we have been able to acquire incredibly beautiful high-resolution 3D images of the malaria parasite infection process of erythrocytes (image 1). Malaria research is outside of my field of expertise, but I’m impressed with the beautiful images and ingenuity of life embodied in malaria parasites. I’m excited about the future progress of collaborative research, including the relationship between the young researchers of both divisions and Nikon staff.
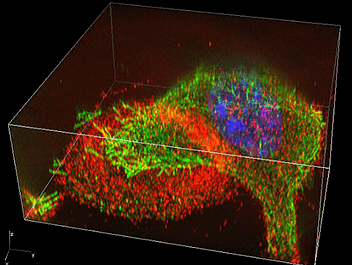
I am researching bone cells, including osteoblasts and osteoclasts, and I believe that the N-SIM provides a new viewpoint for studying the metabolisms of bone cells.
For example, there are ring-like actin-enriched structures called actin rings on the surface of osteoclasts that adhere to bone. Previously, the podosomes, the minute components of this actin ring, could only be observed by electron microscopy. I was surprised that each of these podosomes could be clearly and separately observed using the N-SIM. This is due to the superior performance of super-resolution microscopes. Some objects that could previously only be observed by electron microscopes are now becoming observable by optical microscopes.
N-SIM enables real-time observation of changes in the structure and sequence of podosomes, so we can now discuss the functional changes of osteoclasts based on dynamic analysis of minute structures (image 2).
An additional advantage of the N-SIM is that various types of fluorescent dyes can be used. This has many benefits, since we can observe the samples that we already have, such as common fluorescent immunostaining tissue and fusion proteins joining fluorescent proteins, etc.
The N-SIM also has faster data processing, enabling unrestricted live imaging. Moreover, it renders points that are distant from the surface of the cover glass observable at high resolutions. Three-dimensionally reconstructed images acquired using N-SIM exhibit remarkable quality. I am so excited that we can now observe the multi-dimensional dynamics of minute cell structures that were not previously observable. This has inspired me to open up new fields of research in my work.
The A1+ Confocal Microscope is one of the most popular items of shared equipment at the Division of Analytical Bio-Medicine in ADRES, for its high performance as well as its convenience and easy operability. In addition, the recently installed NIS-Elements C-ER imaging software enables us to observe minute structures using the A1+ at high resolutions close to those of the N-SIM. Furthermore, image stitching enables acquisition of entire images of osteoclasts up to the hundreds of micrometers, allowing multi-scale analysis in which we can select an observation point in a large field-of-view image and magnify that part of the image.
The performance of the A1+’s detectors has greatly improved, resulting in reduced imaging time and simplified parameter setting. Setting imaging conditions to acquire the desired contrast is now easy for any researcher to do. While high-spec microscopes such as super-resolution microscopes and multiphoton microscopes are attracting attention, I appreciate increased specifications that evoke the evolution of confocal microscopes. The evolution of confocal microscopes with superior agility, usability and cost performance will continue to draw an increasing amount of attention.
What are the outstanding aspects of Nikon and Nikon products?
The first advantage is their simple operation. Operating procedures can be easily understood by simply opening the imaging software. The software has a hierarchical operating structure between standard and advanced levels, which is advantageous when explaining the use of the systems at ADRES.
Next, I was impressed by the stunningly clear images of extremely minute structures that Nikon’s DIC microscopes can acquire. In my bone research, pathological conditions and homeostasis of bones can be investigated by observing minute growth rings and the porous structure in bone tissue sections. Generally, there is a trade-off between resolution and contrast in DIC images, but I greatly admire Nikon’s advanced technologies for enabling the acquisition of images that maintain a balance between both factors.
Finally, I appreciate Nikon’s support. They suggest and provide a large variety of solutions with their products, including objectives for diverse applications. I appreciate Nikon’s broad range of technologies and courteous customer support, especially the excellent communication of Nikon’s staff, which helps deepen our understanding and appreciation of microscopes.
What are the future demands for microscopes?
I think multiscale applications will be essential in future microscopy.
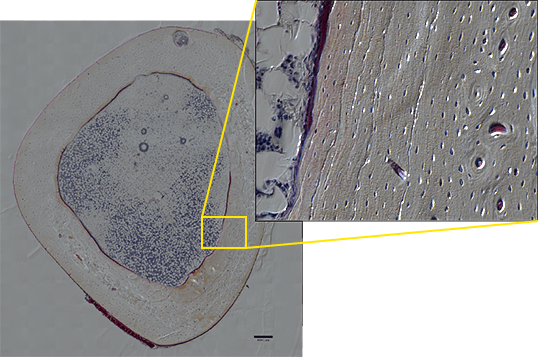
For example, when enlarging the entire image of a bone section created by stitching DIC images together, you can observe the layered structure and growth rings of the bone, and even the network of bone cells. The observed phenomenon can thus be displayed in a more compelling way (image 3). It would be ideal if, in the future, the same point of a sample can be observed from organ to tissue level and even to the molecular level using one system, and I am anticipating the development of microscopes that enable seamless observation in such applications.
Please tell us about the future applications of microscopes.
Super-resolution microscopy reveals that nano-size proteins that were thought to bind themselves to each other in past biochemical experiments, in fact dynamically alternate between bonding and dissociation in a living organism. The dynamics of molecules in living organs are non-uniform, and this imparts non-uniformity to the functions of cells and great influence on the entire organism. In the future, there will be increasing demand for microscopes to provide precise quantitative data concerning fluctuations in molecules in order to acquire more accurate insights into the mechanisms of illnesses as well as whole organisms. Consistencies in variations and noise are the essence of life. I believe that the important role of microscopes in providing new viewpoints on the nature of life will continue to grow.
