Atsushi Miyawaki, M.D., Ph.D.
Senior Team Leader, Laboratory for Cell Function Dynamics, RIKEN Brain Science Institute
尼康设备
- AZ-C1 macro confocal microscope system
- 1x objective lens (AZ-Plan Apo, NA 0.1, W.D. 35 mm)
- 2x objective lens (AZ-Plan Fluor, NA 0.2, W.D. 45 mm)
Please tell us about the development of light imaging and scale.
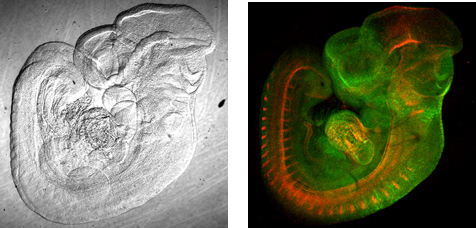
I have long believed that there is much information to be obtained from the intermediate stage between a biochemical approach such as cell componential analysis using electrophoresis and optical imaging of living specimens—for example, from research into fixed specimens. In particular, fluorescent protein technology and gene induction technologies have enabled the selective labeling of nerve cells regardless of depth; however, there is as yet little technology for visualizing the neural networks running from the surface to the depths of the brain together. The Scale reagent we have developed can make biological tissue transparent like jelly. This has enabled deep imaging without damage to the specimen.
Dr. Hiroshi Hama focused attention on the fact that the PVDF membrane used for transferring proteins in western blotting becomes transparent in high-concentration urea solution. In addition, we were already aware that most fluorescent proteins were urea-resistant, though high-concentration urea solution is generally used for protein denaturation. The impetus for the Scale project was the belief that tissue clearing technology could probably be developed based on urea. Initially, it took quite a long time for the entire brain of a mouse to become transparent when soaked in our early reagents. However, our capacity for patience seems to have paid off. The Scale technology was improved and finally the ScaleA2 reagent was completed. We now know that making a cut in the mouse brain makes it become transparent more rapidly. Two or three hours may be enough for a brain tissue block.
We have also focused attention on the biological debate over whether the neural stem cells in the dentate gyrus of the hippocampus lie close to the blood vessels. Through the observation of BrdU-labeled neural stem cells and immunostained blood vessels, numerous researchers, in their papers, have concluded that they are close. However, the data obtained from the observation of sliced tissue is not very persuasive, and they could be criticized for choosing only favorable data. It is necessary to measure the distances between the neural stem cells and the blood vessels in three-dimensional space over the wide area of the dentate gyrus of the hippocampus. We solved this problem with Scale technology. Furthermore, for identification of neural stem cells, our Fucci technology was used.

What part did the AZ-C1 macro confocal microscope system play?
A wide range of applications are possible when used in combination with Scale technology.
For example, if neurons fire, the transcription of immediate-early genes such as c-fos and Arc is enhanced. Therefore, neuronal firing can be monitored with the fluorescence signal of a reporter gene of fluorescent protein such as Venus located downstream of a promoter that controls c-fos and Arc expression. Quantifying this signal by capturing a number of slices of the brain and reconstructing three-dimensional images entailed tremendous effort. Using the AZ-C1, however, we proved that it is possible to simplify the quantification by projecting three-dimensional information of the brain, made transparent with Scale, to construct a two-dimensional image.
The imaging depth limit with single-photon excitation is generally only around 0.2 mm due to the influence of scattered light, while it is 0.8 mm with two-photon excitation. As Scale technology minimizes light scattering in the sample, it is more effective when used with a single-photon excitation microscope. It may also be beneficial for the AZ-C1.
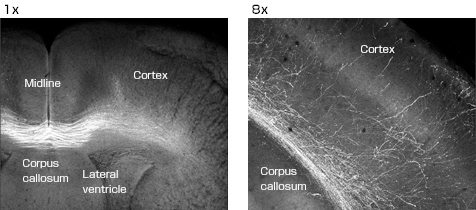
For example, if the YFP gene is administered to the cerebral cortex of the right hemisphere of the brain of an embryonic mouse, it is observed that axons project into the left side of the cerebral cortex across the corpus callosum during nervous system development. The AZ-C1 is effective in this kind of research, in which wide-area images must be obtained.
What would you like future microscopes and imaging equipment to feature?
It would be ideal if there were a microscopic imaging system that could freely zoom in and out.
I want to see our fluorescent-imaging technology and Scale technology make a major contribution to human pathological diagnosis. I am interested in the establishment of the technology for use in finding lesions in large tissue mass. I believe that Scale technology will contribute to medical science, as well as to academic disciplines such as developmental biology. I also expect AZ-C1 to make a contribution.
On Nikon
Professor Roger Tsien (2008 Nobel laureate for chemistry), who has conducted numerous joint research and development projects with Nikon, was my adviser. Therefore, I have a long-standing relationship with Nikon. I hope that the company will develop products that challenge researchers.